Latent catalytic curing agents
A curing agent, latent technology, used in the field of catalytic curing agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
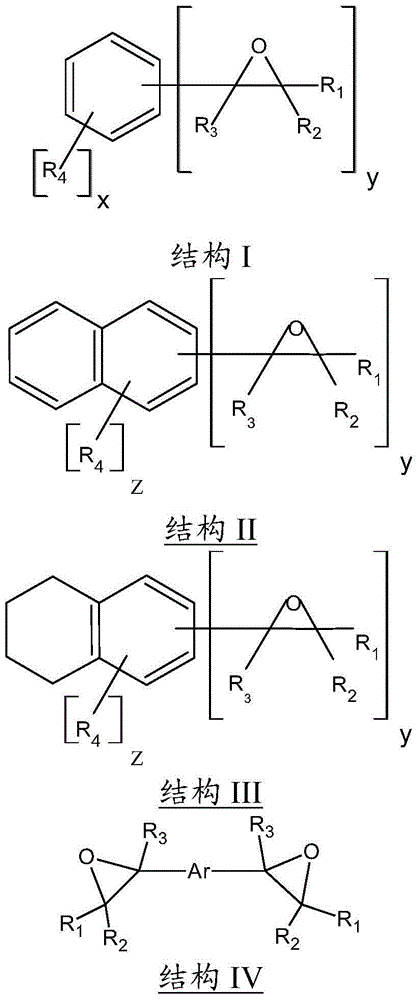
[0023]
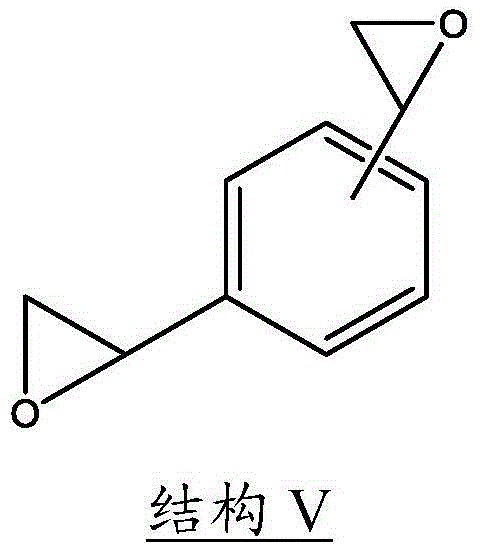
[0024] When DVBDO is prepared by methods known in the art, it is possible to obtain one of three possible isomers: ortho, meta, and para. Accordingly, the present invention includes DVBDOs illustrated by any one of the above structures individually or as a mixture thereof. Structures VI and VII above show the meta (1,3-DVBDO) and para (1,4-DVBDO) isomers of DVBDO, respectively. The ortho isomer is rare; DVBDO is generally produced in most cases in a ratio of meta (structure VI) to para (structure VII) isomers in the range of 9:1 to 1:9. As an embodiment, the present invention preferably includes a ratio of structure VI to structure VII in the range of 6:1 to 1:6, and in other embodiments, the ratio of structure VI to structure VII may be in the range of 4:1 to 1 :4 or 2:1 to 1:2 range.
[0025] In yet another embodiment of the present invention, the divinylarene dioxide may contain a certain amount (eg, less than 20% by weight) of substituted arenes and / or arene ...
Embodiment
[0060] The following catalysts were used in the examples: Cycat 600 (70 wt% solution of dodecylbenzenesulfonic acid in isopropanol, commercially available from Cytec, Inc.), methyl p-toluenesulfonate (MPTS), Methyl methanesulfonate (MMS), methyl trichloroacetate (MTCA), methyl trifluoroacetate (MFTA), and tetraethylmethylene diphosphonate (TEMDP).
[0061] In the following examples, the glass transition temperature (T g ) by differential scanning calorimetry (DSC) using a temperature scan rate of 10 °C / min to measure the temperature at half height of the heat flow curve or by thermodynamic analysis (TMA) using a temperature scan rate of 10 °C / min to measure the outer dimension of the curve The temperature at the starting point is measured; and the formulation viscosity is measured using 10s -1 Shear rate and temperature were measured on a parallel plate rheometer run at 25 °C.
[0062] Examples 1–3
[0063] Examples 1-3 illustrate the curing of DVBDO with latent catalytic...
Embodiment 4 and 5 and comparative example A
[0068] Examples 4 and 5 and Comparative Example A illustrate curing of DVBDO with latent catalytic curing agents MPTS, MMS and Cycat 600, respectively. Add 25 g of DVBDO and 0.05–0.06 g of a latent catalytic curing agent (MPTS, MMS, or Cycat 600) to an 8 oz (237 mL) jar. The formulations were mixed at room temperature, poured into 6"x 6"x 0.125" (15.2x15.2x0.32cm) aluminum molds, and placed in an air circulating oven at 60°C, 70°C, 80°C, 90°C , 100°C, 105°C, 110°C, 115°C, 120°C, 120°C, and 150°C for 30 minutes. The samples were then post-cured for 30 minutes at 160°C, 170°C, 185°C, 200°C, and 225°C, respectively. The resulting thermoset was analyzed by TMA analysis and the results of the analysis are described in Table II.
[0069] Table II
[0070]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com