Method for synthesizing p-hydroxypropiophenone
A technology of p-hydroxybenzene and a synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of a large number of catalysts, low propionic acid reaction activity, no reports on yields, etc., and achieves easy follow-up treatment of the reaction, and cheap and easy-to-obtain catalysts. , post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the synthesis of p-hydroxyacetophenone
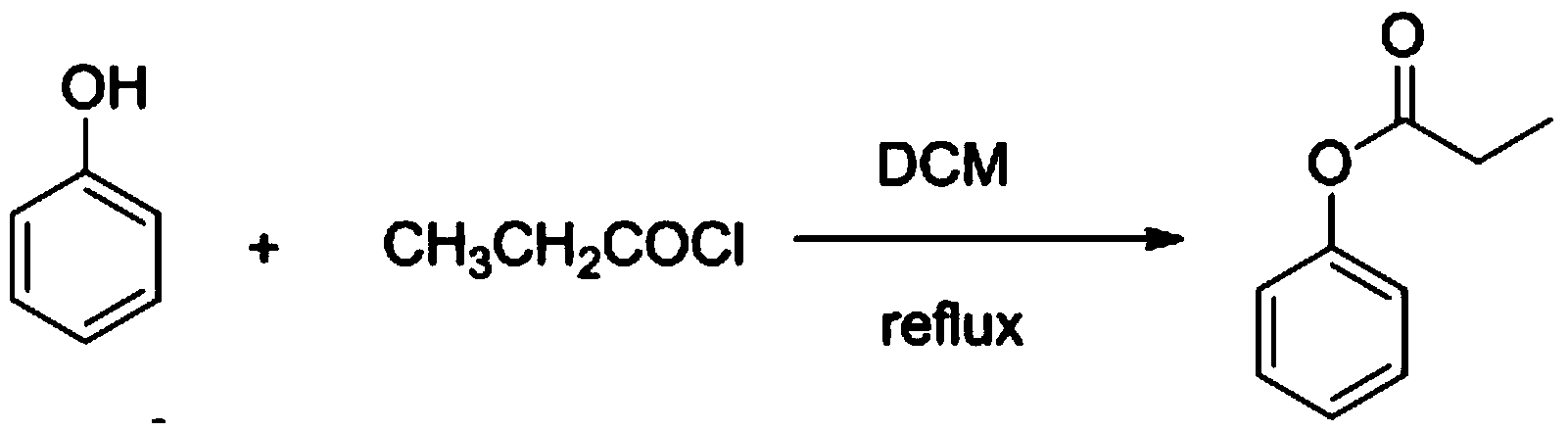
[0022] 1) Preparation of Propionylphenol
[0023] Phenol (10.0g, 0.106mol) was added to 30ml of dichloromethane, sodium bicarbonate (0.8904g, 10.6mmol) was added, propionyl chloride (12.0g, 0.129mol) was added dropwise under heating and reflux, and the reaction was refluxed for 4h under stirring . TLC monitors that the reaction is completed, the reaction solution is poured into 100ml ice water, and the 3 The pH of the solution was adjusted to 7, the organic phase was separated, and the aqueous phase was extracted twice with 50 ml of dichloromethane, the organic phases were combined, dried, evaporated at normal pressure, and the organic solvent was recovered to obtain 13.2 g of the product with a yield of 82.8%. .
[0024] 2) preparation of p-hydroxypropiophenone
[0025] Propionylphenol (8.0g, 0.052mol) was added to methanesulfonic acid (14.0ml, 0.212mol), the temperature was controlled at 30°C, and the reaction...
Embodiment 2
[0026] Embodiment 2: the synthesis of p-hydroxyacetophenone
[0027] 1) Preparation of Propionylphenol
[0028] Phenol (10.0g, 0.106mol) was added to 30ml of dichloroethane, sodium bicarbonate (0.89g, 10.6mmol) was added, propionyl chloride (13.8g, 0.148mol) was added dropwise under heating and reflux, and the reaction was reflux under stirring 5h. TLC monitors that the reaction is completed, and the reaction solution is poured into 100ml ice water, and the 2 CO 3 The pH of the solution adjustment system was 8, the organic phase was separated, and the aqueous phase was extracted three times with 50 ml of dichloroethane, the organic phases were combined, dried, evaporated at normal pressure, and the organic solvent was recovered to obtain 13.9 g of the product with a yield of 87.5%. .
[0029] 2) preparation of p-hydroxypropiophenone
[0030] Propionylphenol (8.0g, 0.052mol) was added to methanesulfonic acid (14.0ml, 0.212mol), the temperature was controlled at 50°C, and t...
Embodiment 3
[0031] Embodiment 3: the synthesis of p-hydroxyacetophenone
[0032] 1) Preparation of Propionylphenol
[0033]Phenol (10.0g, 0.106mol) was added to 30ml of chloroform, sodium bicarbonate (0.8904g, 10.6mmol) was added, propionyl chloride (15.7g, 0.170mol) was added dropwise under heating and reflux, and the reaction was refluxed for 6h under stirring . TLC monitors that the reaction is completed, the reaction solution is poured into 100ml ice water, and the 3 The pH of the solution was adjusted to 7, the organic phase was separated, and the aqueous phase was extracted three times with 50 ml of chloroform. The organic phases were combined, dried, evaporated under normal pressure, and the organic solvent was recovered to obtain 14.2 g of the product with a yield of 88.8%.
[0034] 2) preparation of p-hydroxypropiophenone
[0035] Propionylphenol (8.0g, 0.052mol) was added to methanesulfonic acid (14.0ml, 0.212mol), the temperature was controlled at 50°C, and the reaction was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com