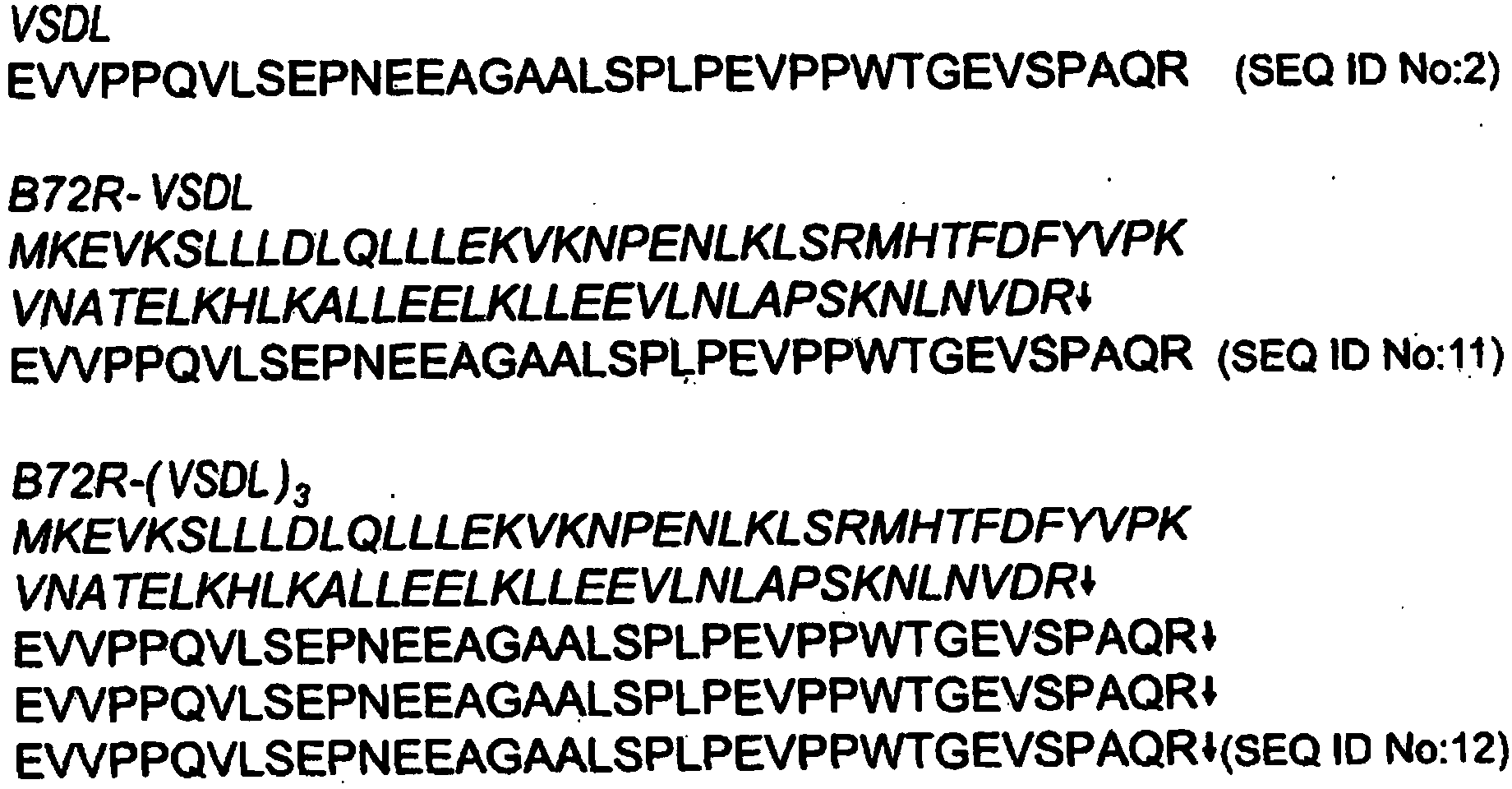Method of producing a recombinant peptide
A technology for recombinant peptides and peptide cutting, applied in recombinant DNA technology, chemical instruments and methods, peptides, etc., and can solve the problems of expensive synthesis and preparation of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Embodiment 1 prepares VSDL in escherichia coli
[0179] The amino acid sequence of human VSDL (designated VSDL in the Examples) is:
[0180] Glu-Val-Val-Pro-Pro-Gln-Val-Leu-Ser-Glu-Pro-Asn-Glu-Glu-Ala-Gly-Ala-Ala-Leu-Ser-Pro-Leu-Pro-Glu-Val- Pro-Pro-Trp-Thr-Gly-Glu-Val-Ser-Pro-Ala-Gln-Arg
[0181] (SEQ ID NO:2).
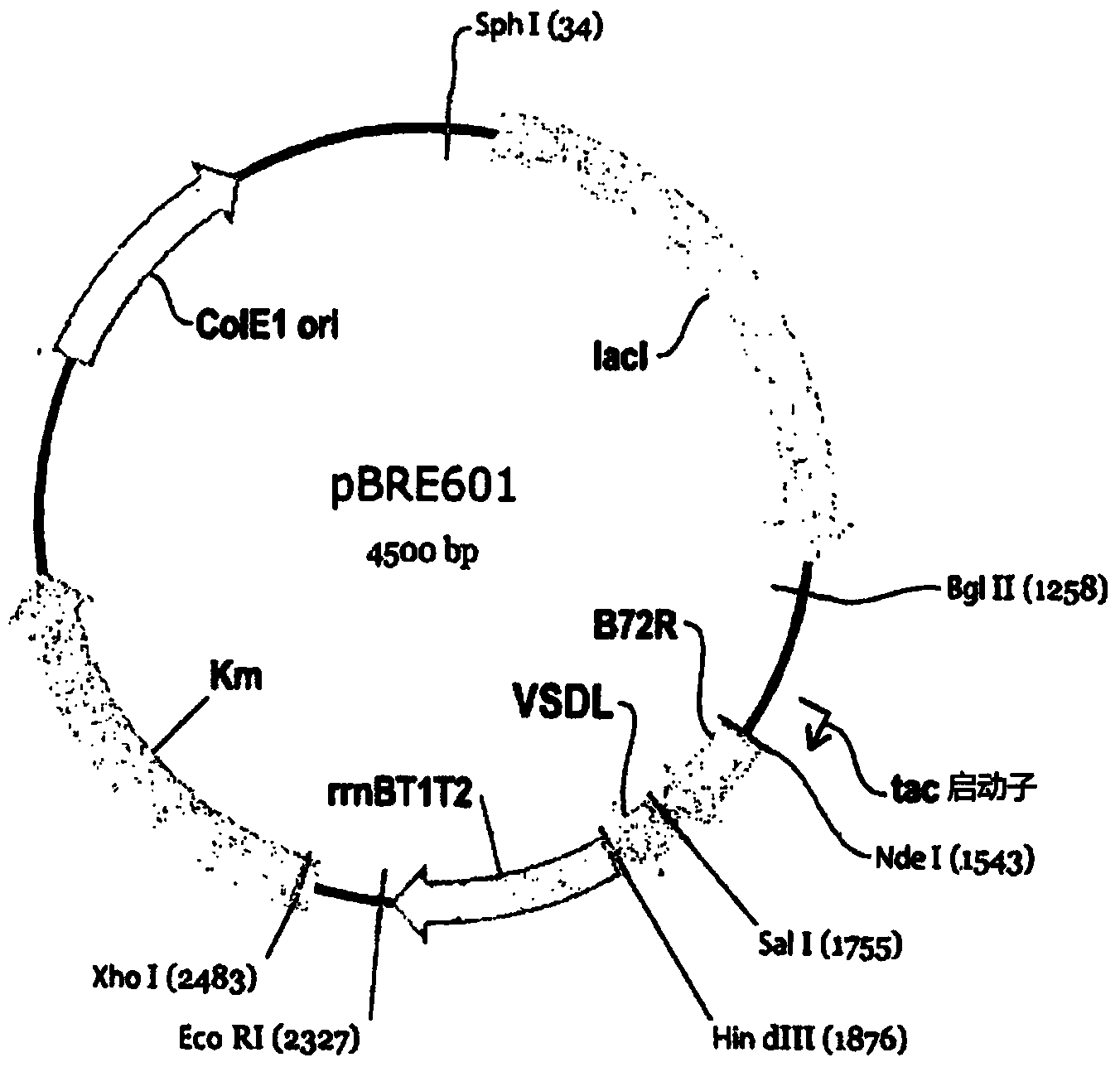
[0182]In this example, the fusion polypeptide approach was utilized in an attempt to attempt the absence of internal arginine (R) and lysine (K) residues in the polypeptide sequence, and the presence of a trypsin cleavage site conveniently provided at the C-terminus Arginine (R) strategy for the study of recombinant expression of VSDL in Escherichia coli ( figure 1 ).
[0183] Preparation of expression constructs
[0184] First, two fusion peptides are designed:
[0185] (i) a single VSDL sequence (designated B72R-VSDL and also referred to herein as "1-mer") fused to a 73 amino acid fusion partner designated B72R, and
[0186] (ii) Three VSDL repeats fu...
Embodiment 2
[0223] Example 2 Recombinant preparation of VSDL 10-mer in Escherichia coli
[0224] Lysates were produced from fermentations of the BR067 E. coli strain described in Example 1 containing the pBRE606 expression construct encoding 10 tandem copies of the VSDL peptide as described in the section "Fed-batch fermentation" of Example 1. B72R-(VSDL) 10 Fusion polypeptides (ie 10-mers).
[0225] Thaw 275 mL of the 10-mer lysate and adjust the pH to pH 8.0 with 1M NaOH. (As described in Example 1) was added to the fusion polypeptide at a mass ratio of 1:120, and digestion was carried out at room temperature for 2 hours. The pH of the digested lysate was adjusted to pH 4.4 with 1M acetic acid to precipitate impurities, and then filtered with a Millistak+Pod Labscale DOHC filter (MD0HC027H2) 0.027m 2 Depth filtering.
[0226] The filtrate was purified by direct loading onto a reverse phase Amberchrom CG300C resin column. The column was flushed with about 15 column volumes of 0.5% ...
Embodiment 3
[0232] Example 3 Recombinant preparation of VSDL 3-link in Escherichia coli
[0233] VSDL lysates were obtained as per Example 2, except that the BR067 E. coli strain described in Example 1 was used containing the pBRE602 expression construct encoding B72R-(VSDL) with 3 tandem copies of the VSDL peptide 3 Fusion polypeptides (ie 3-mers).
[0234] The B72R-(VSDL) 3 The lysate (175 mL) was thawed to a temperature between 15 and 25°C. The pH of the lysate was adjusted to 8.0±0.1 with 1M NaOH. Reconstituted TrypZean was added at 25 μg / mL lysate. Digests were mixed gently for 5 min and then allowed to stand overnight at room temperature. The pH of the digest solution was adjusted to 4.4 ± 0.1 with 1M. The solution was gently stirred using a magnetic stirrer.
[0235] Then pass through Millistak+Pod Labscale D0HC filter (MD0HC027H2) 0.027m 2 The lysate was depth filtered at 4.2 mL / min and then loaded directly onto an Amberchrom CG300C column (16 mm ID column, bed height 7.0 c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com