A herpes simplex virus thymidine kinase mutant and its preparation method and use
A technology of herpes simplex virus and thymidine kinase, which is applied in the field of genetic engineering, can solve the problems of large side effects and poor curative effect, and achieves the effect of enhancing the effect opportunity, good clinical application prospect and reducing the competitive interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
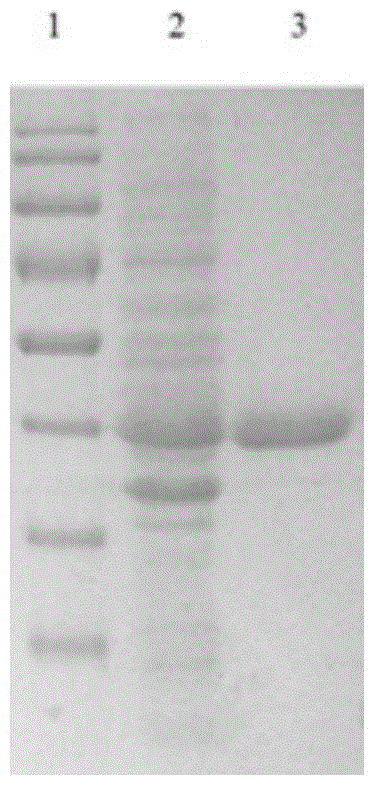
[0030] Embodiment 1 Preparation of herpes simplex virus thymidine kinase mutant of the present invention
[0031] 1. Recombinant expression
[0032] 1.1 Gene cloning
[0033] a. Synthetic recombinant target gene:
[0034] (1) Natural tk gene: chemical synthesis method synthetic GenBank number is the HSV-1tk gene of AB032875, and its nucleotide sequence is as shown in SEQ NO: 1:
[0035]
[0036] (2) tk gene variant 1: the 160th amino acid is mutated from isoleucine (I) to leucine (L) by chemical synthesis method, and the 161st amino acid is mutated from phenylalanine (F) to leucine Acid (L), the 167th position is mutated from alanine (A) to tyrosine (Y), and the 168th position is mutated from alanine (A) to tyrosine (Y) 4 amino acid residues are mutated simultaneously Mutant TK (I160L, F161L, A167Y, A168Y), abbreviated as mtk1608, its nucleotide sequence is shown in SEQ NO: 2:
[0037]
[0038]
[0039] Its amino acid sequence is shown in SEQ NO: 4:
[0040]
...
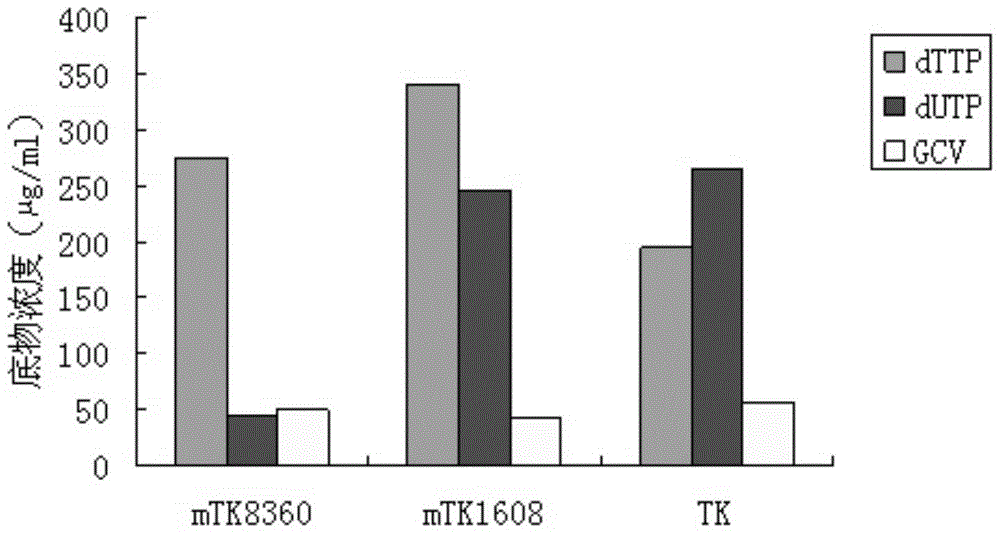
Embodiment 2
[0080] Construction of embodiment 2 pBES-mtk1608
[0081] 1. Experimental method
[0082] (1) Preparation of pBES vector
[0083]The first step is to replace the sequence region of the Ampicillin resistance gene of the pGEX-5x-1 plasmid 1344-2220nt with the kanamycin resistance gene, that is, (a) first use the PCR method from the plasmid pEASY-T1 whose GenBank number is EU233623 Amplify the 1203-2114nt fragment to obtain the kanamycin resistance gene. The forward PCR primer is: agtagacgtcctggtaag gttgggaag, the position is at 1203-1219nt, and an AatII restriction site is added at the 5' end. The reverse primer is: acgtcagtggctgcaattcagaagaactcgtc, the position is at 2114-2085nt, with an AlwN1 restriction site added at the 5' end; the PCR amplification conditions are: 95°C for 4min, followed by 25 cycles of 95°C for 40Sec, 55°C for 30Sec, and 72°C for 1min; (b) the above PCR purified product and pGEX-5x-1 plasmid were double-digested with Aat II and AlwN1 respectively, and th...
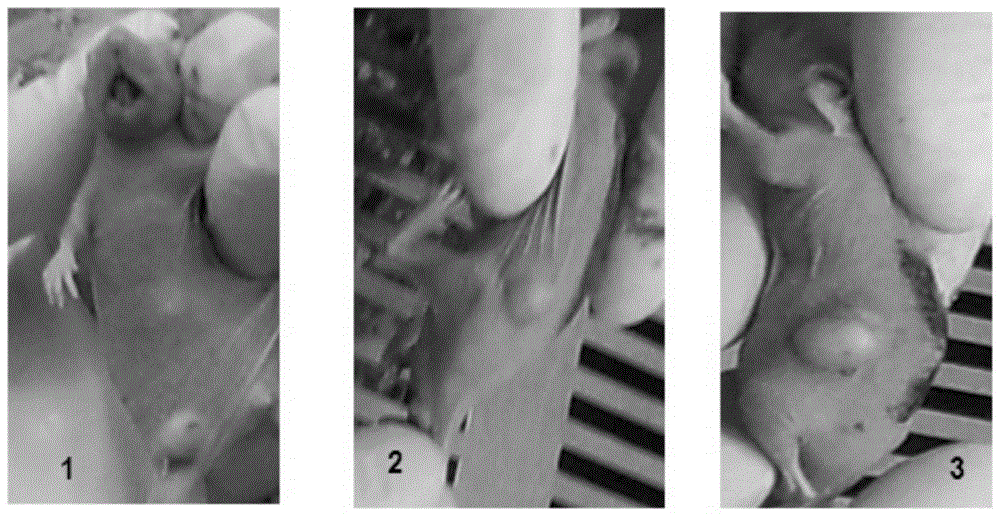
Embodiment 3
[0089] Embodiment 3 The preparation of the recombinant bifidobacterium containing pBES-mtk1608 recombinant vector
[0090] The recombinant bifidobacterium of the pBES-mtk1608 recombinant vector prepared in Example 2 (also known as Bifidobacterium-mTK1608) can be prepared as an injection for vacuum safe packaging at room temperature and for injection, and is constructed according to the following steps:
[0091] a) The recombinant bifidobacterium of the pBES-mtk1608 recombinant vector prepared in Example 2 (i.e., the bifidobacterium-mTK1608 solid tumor gene therapy system), which was preserved after freeze-drying with 20% skimmed milk in a vacuum, was Bifidobacterium-mTK1608 Seed bacteria for gene therapy systems for solid tumors.
[0092] b) Resuscitate the seed bacteria described in a) above, spread it on the resistant MRS medium containing kanamycin and culture it anaerobically for 48 hours, then pick a single resistant colony and culture it in the resistant MRS liquid Bact...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com