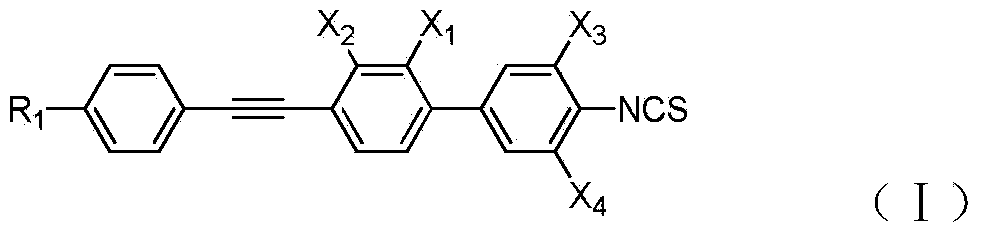
Liquid crystal compound with high birefringence and wide nematic phase temperature interval and composition comprising liquid crystal compound
A technology for liquid crystal compounds and liquid crystal compositions, applied in the fields of organic chemistry, liquid crystal materials, chemical instruments and methods, etc., can solve the problems of inducing smectic phase, poor compatibility, easy to appear crystallization phenomenon, etc., and achieve wide nematic phase. Temperature range, effect of high birefringence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
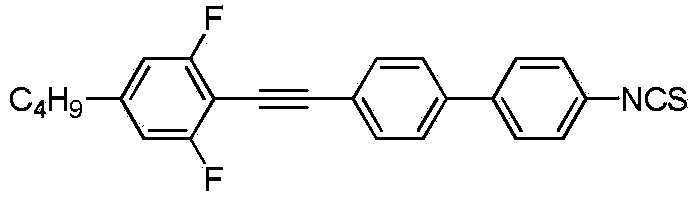
[0026] Preparation of 4'-((4-ethylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanate-1,1'-biphenyl:
[0027] The specific structural formula is as follows:
[0028]
[0029] The preparation process is as follows:
[0030] (1) Under nitrogen protection, add 13.1g (98mmol) of p-bromoiodobenzene, 100mL of tetrahydrofuran, 31.5mL of triethylamine (0.2mol), PdCl 2 (PPh 3 ) 2 0.7g (0.001mol), CuI 0.6g (0.003mol). Then, 27.6 g (98 mmol) of p-ethylphenylacetylene and 50 mL of tetrahydrofuran were added to the constant pressure dropping funnel. Nitrogen was replaced three times, the temperature of the system was lowered to -5°C to 0°C, and a tetrahydrofuran solution of ethylphenylacetylene was added dropwise. After the dropwise addition was completed, it was raised to room temperature and stirred overnight. The reaction solution was filtered to remove a large amount of salt. The filtrate was spin-dried and passed through a silica gel column with n-heptane. Rotate the liquid throu...
Embodiment 2
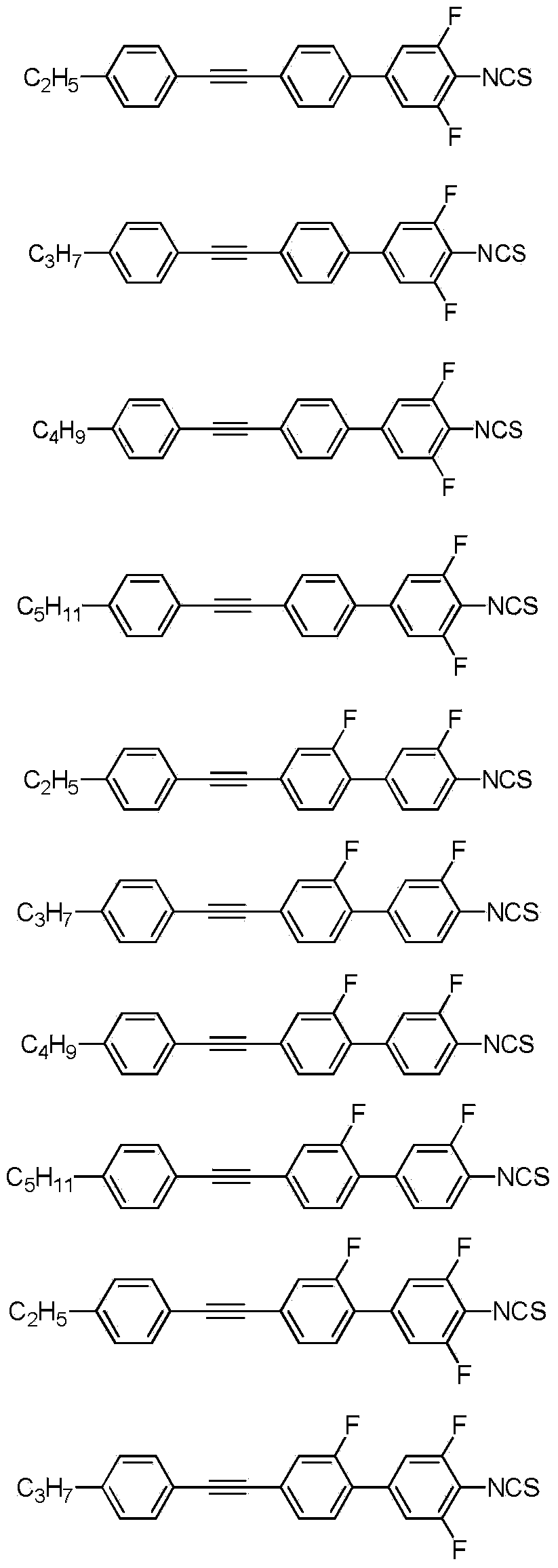
[0038] Using propylphenylacetylene instead of ethylphenylacetylene in Example 1, the same method was used to synthesize 4'-((4-propylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanate Ester-1,1′-biphenyl. Structure Identification:
[0039] 1 H NMR (δ, CDCl 3): 0.914~0.963(t, 3H), 1.579~1.703(m, 2H), 2.567~2.617(t, 2H), 7.135~7.163(d, J=14Hz, 4H), 7.416~7.459(m, 4H) , 7.536~7.564 (d, J=14Hz, 2H). IR (KBr, cm -1 ): 3028 (w, Ar-H), 2960, 2870 (m, -CH 3 ), 2929 (w, -CH 2 ), 2218 (w, c≡c), 2035 (s, NCS), 1623, 1547, 1508 (m, Ar), 1438, 1206, 1113, 1042, 945, 831.
[0040] The above structural identification data show that the synthesized compound is indeed 4′-((4-propylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanate-1,1′-biphenyl .
[0041] 4'-((4-Propylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanato-1,1'-biphenyl was tested by DSC at a temperature increase of 5°C / min The liquid crystal phase transition temperature, the result is: C 90.30N 233.76I, melting enthalpy value 21.47KJ·m...
Embodiment 3
[0043] Using butylphenylacetylene instead of ethylphenylacetylene in Example 1, the same method was used to synthesize 4'-((4-butylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanate Ester-1,1′-biphenyl. Structure Identification:
[0044] 1 H NMR (δ, CDCl 3 ): 0.895~0.943(t, 3H), 1.255~1.377(m, 2H), 1.485~1.628(t, 2H), 2.568~2.619(t, 2H), 7.065~7.187(m, 4H), 7.368~7.394 (m, 4H), 7.483-7.510 (m, 2H). IR (KBr, cm -1 ): 3026 (w, Ar-H), 2960, 2858 (m, -CH 3 ), 2925 (w, -CH 2 ), 2211 (w, c≡c), 2051 (s, NCS), 1623, 1550, 1507 (m, Ar), 1441, 1204, 1113, 1042, 943, 830.
[0045] The above structural identification data show that the synthesized compound is indeed 4′-((4-butylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanate-1,1′-biphenyl .
[0046] 4'-((4-butylphenyl)ethynyl)-3,5-difluoro-4-isothiocyanate-1,1'-biphenyl was tested by DSC at a temperature increase of 5°C / min The liquid crystal phase transition temperature, the result is: C 68.08N 232.92I, and the nematic phase temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com