Preparation method of bismuth subcarbonate microflowers and product
A technology of bismuth oxycarbonate and micro-flowers, which is applied in chemical instruments and methods, bismuth compounds, inorganic chemistry, etc., can solve the problems of poor controllability of morphology, disordered flower-like structure, and low crystallinity of bismuth oxycarbonate micro-flowers. , to achieve the effect of easy control, simple preparation process and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
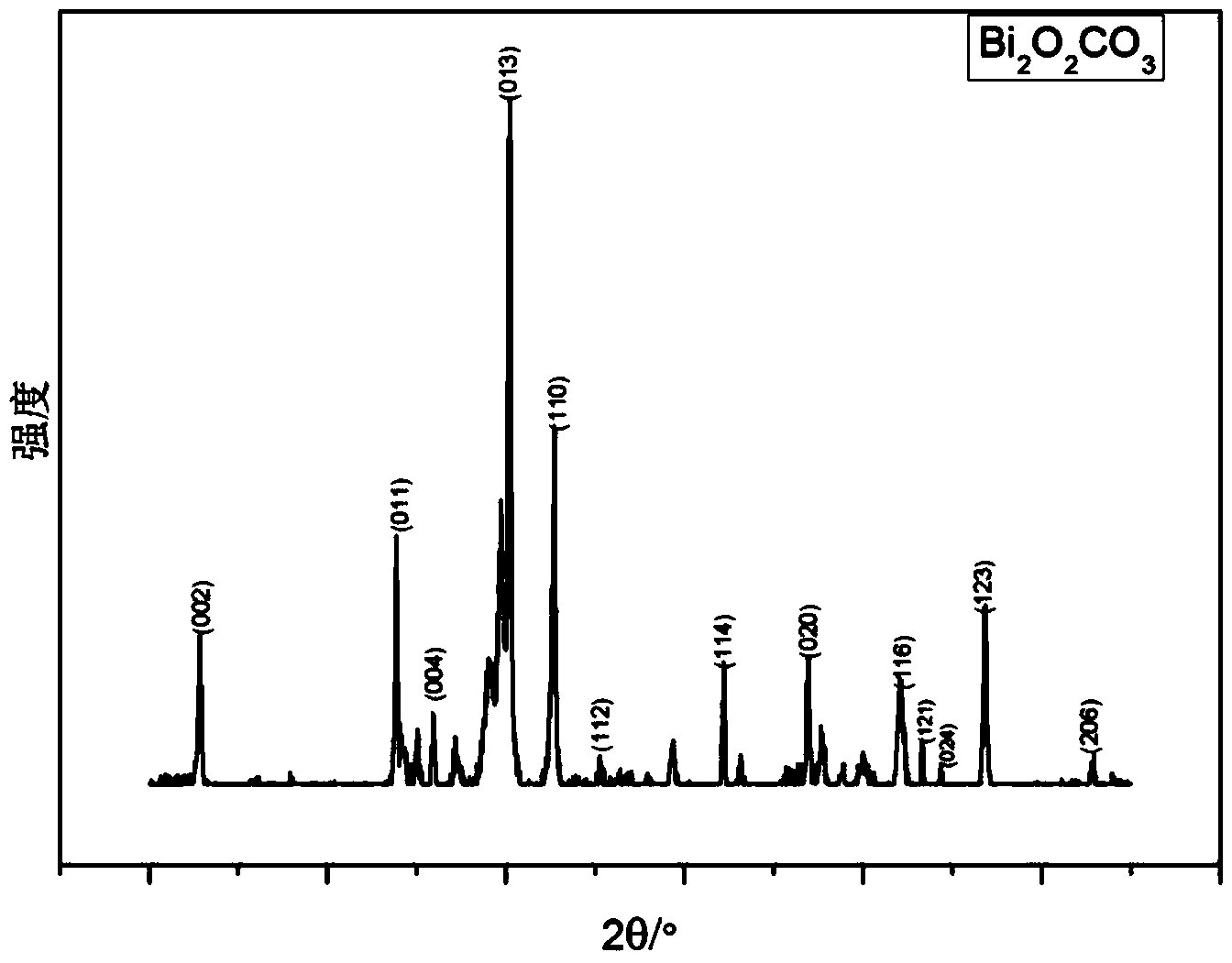
Image
Examples
Embodiment 1
[0026] 1) Weigh 8 mmol of tetrabutyl titanate, add it dropwise into deionized water, and control the concentration of tetrabutyl titanate to 1.0 mol / L.
[0027] 2) Weigh 11 mmol of bismuth nitrate pentahydrate, add it into the suspension prepared in step 1), and stir thoroughly.
[0028] 3) Weigh 0.04 mol of potassium hydroxide, add to the suspension solution prepared in step 2), and stir for at least 30 minutes.
[0029] 4) Weigh 28 mmol of ammonium citrate, dissolve it in deionized water, and control the concentration of ammonium citrate to 2.8 mol / L.
[0030] 5) Add the solution prepared in step 4) dropwise to the suspension prepared in step 3) under stirring state, and disperse through sufficient stirring and ultrasonic vibration.
[0031] 6) Add the suspension prepared in step 5) into the liner of the reaction kettle. Use deionized water to adjust its volume to account for 4 / 5 of the inner tank volume of the reactor to obtain a suspension of the reaction precursor. At ...
Embodiment 2
[0034] 1) Weigh 10 mmol of tetrabutyl titanate, add it dropwise to deionized water, and control the concentration of tetrabutyl titanate to 1.0 mol / L.
[0035] 2) Weigh 13 mmol of bismuth nitrate pentahydrate, add it into the suspension prepared in step 1), and stir thoroughly.
[0036] 3) Weigh 0.04 mol of potassium hydroxide, add to the suspension solution prepared in step 2), and stir for at least 30 minutes.
[0037] 4) Weigh 28 mmol of ammonium citrate, dissolve it in deionized water, and control the concentration of ammonium citrate to 2.8 mol / L.
[0038] 5) Add the solution prepared in step 4) dropwise to the suspension prepared in step 3) under stirring state, and disperse through sufficient stirring and ultrasonic vibration.
[0039] 6) Add the suspension prepared in step 5) into the liner of the reaction kettle. Use deionized water to adjust its volume to account for 4 / 5 of the inner tank volume of the reactor to obtain a suspension of the reaction precursor. At t...
Embodiment 3
[0042] 1) Weigh 9 mmol of tetrabutyl titanate, add it dropwise into deionized water, and control the concentration of tetrabutyl titanate to 1.0 mol / L.
[0043] 2) Weigh 12 mmol of bismuth nitrate pentahydrate, add it into the suspension prepared in step 1), and stir thoroughly.
[0044] 3) Weigh 0.04 mol of potassium hydroxide, add to the suspension solution prepared in step 2), and stir for at least 30 minutes.
[0045] 4) Weigh 29 mmol of ammonium citrate, dissolve it in deionized water, and control the concentration of ammonium citrate to 2.9 mol / L.
[0046] 5) Add the solution prepared in step 4) dropwise to the suspension prepared in step 3) under stirring state, and disperse through sufficient stirring and ultrasonic vibration.
[0047] 6) Add the suspension prepared in step 5) into the liner of the reaction kettle. Use deionized water to adjust its volume to account for 4 / 5 of the inner tank volume of the reactor to obtain a suspension of the reaction precursor. At ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com