External liniment for treating bone hyperplasia and rheumatic cold-heat diseases and preparation method thereof
A technology for bone hyperplasia and rheumatism, applied to bone diseases, medical preparations containing active ingredients, non-central analgesics, etc., can solve the problems of lack of curative effect, slow action, etc., achieve simple and easy preparation methods, and promote inflammation Disappearance, the effect of high content of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
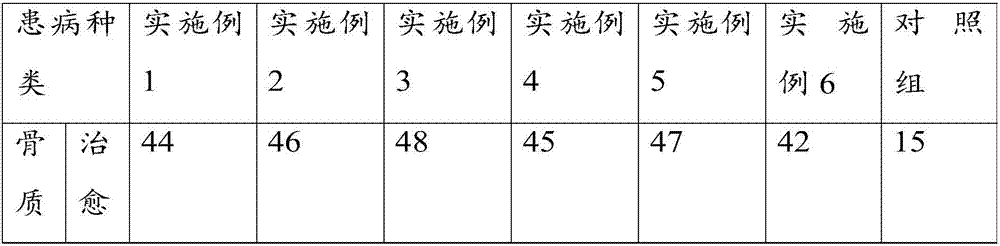
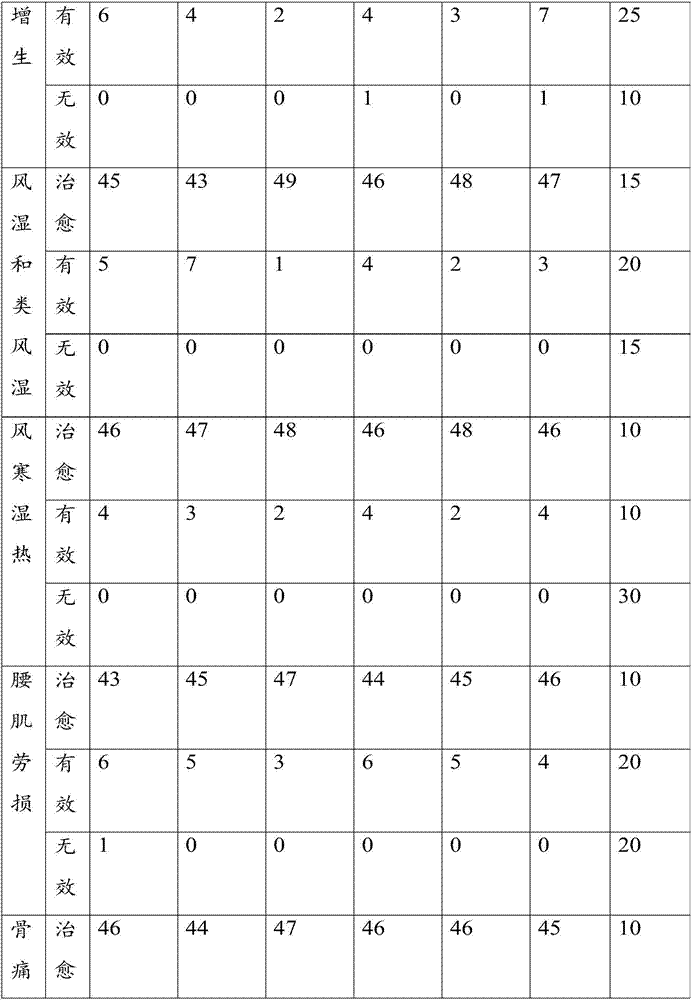
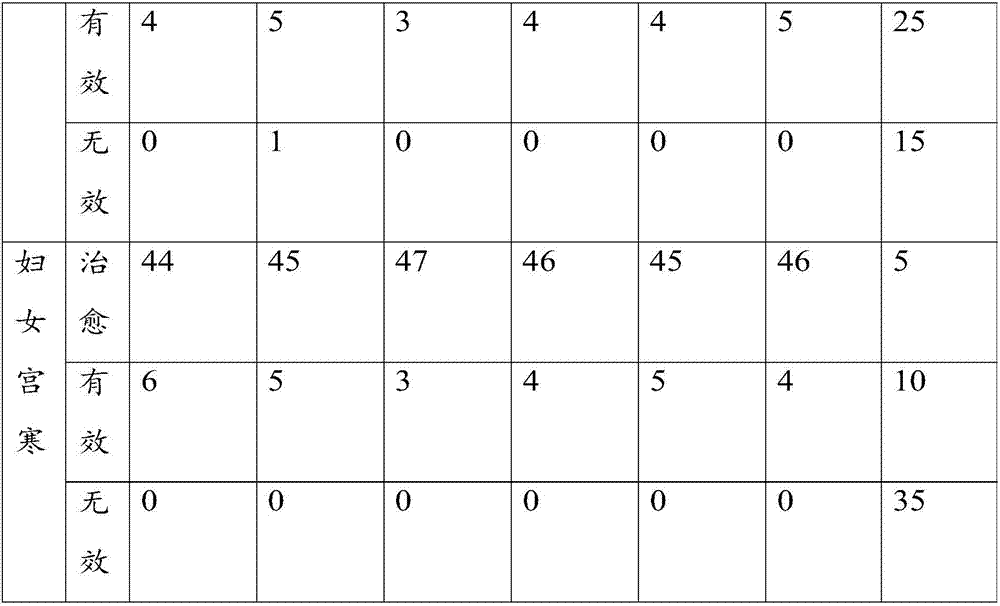
Examples
Embodiment 1
[0071] Take the raw materials of agent A and agent B respectively, wherein, the raw materials of agent A include: in parts by weight, 3 parts of black sheath snake, 7 parts of xugufeng, 5 parts of sumac, 3 parts of camphor, 4 parts of rhubarb, 5 parts of Lingxian, 7 parts of Speranthum, 8 parts of safflower, 5 parts of Sichuan pepper, 8 parts of cinnamon sticks, 3 parts of frankincense, and 8 parts of medlar;
[0072] The raw materials of agent B include: in parts by weight, 3 parts of Dilonga, 3 parts of Fangji, 3 parts of Caulis Spatholobus, 1 part of Evodia rutaecarpa, 3 parts of Xu Changqing, 1 part of Angelica sinensis, 3 parts of Shengchuanwu, 3 parts of Aconitum, 5 parts of Lycium barbarum, 3 parts of Panax notoginseng, 3 parts of notopterygium, 2 parts of myrrh, 3 parts of Shichangpu, 4 parts of Chuanxiong, 3 parts of Asarum, 3 parts of Angelica dahurica, 4 parts of Chuanjiao, 3 parts of Daxueteng;
[0073] The raw materials of agent A and agent B were crushed respecti...
Embodiment 2
[0077] Take the raw materials of agent A and agent B respectively, wherein the raw materials of agent A include: in parts by weight, 5 parts of black scabbard snake, 8 parts of xugufeng, 7 parts of sumac, 5 parts of camphor, 5 parts of Yuanhu, 7 parts of Lingxian, 8 parts of Speranthum, 10 parts of safflower, 7 parts of Sichuan pepper, 9 parts of cinnamon sticks, 5 parts of frankincense, and 9 parts of medlar;
[0078] The raw materials of agent B include: in parts by weight, 5 parts of Dilonga, 5 parts of Fangji, 5 parts of Caulis Spatholobus, 2 parts of Evodia, 5 parts of Xu Changqing, 2 parts of Angelica, 5 parts of Shengchuanwu, 5 parts of Aconitum, 7 parts of Lycium barbarum, 5 parts of Panax notoginseng, 5 parts of Notopterygium, 4 parts of Myrrh, 4 parts of Shichangpu, 4 parts of Chuanxiong, 5 parts of Asarum, 5 parts of Angelica dahurica, 4 parts of Chuanjiao, 5 parts of Daxueteng;
[0079] The raw materials of agent A and agent B were crushed respectively, and the par...
Embodiment 3
[0083] The raw materials of agent A and agent B are taken respectively, wherein the raw materials of agent A include: in parts by weight, 6 parts of black sheath snake, 10 parts of xugufeng, 7 parts of sumac, 5 parts of camphor, 5 parts of Yuanhu, 8 parts of Lingxian, 9 parts of Speranthum, 12 parts of safflower, 8 parts of Sichuan pepper, 9 parts of cinnamon sticks, 6 parts of frankincense, 10 parts of medlar;
[0084] The raw materials of agent B include: in parts by weight, 6 parts of Dilonga, 5 parts of Fangji, 5 parts of Caulis Spatholobus, 3 parts of Evodia rutaecarpa, 6 parts of Xu Changqing, 3 parts of Angelica sinensis, 6 parts of Shengchuanwu, 5 parts of Aconitum, 7 parts of medlar, 6 parts of Panax notoginseng, 6 parts of notopterygium, 5 parts of myrrh, 5 parts of Shichangpu, 4 parts of Chuanxiong, 5 parts of Asarum, 5 parts of Angelica dahurica, 5 parts of Chuanjiao, 6 parts of Daxueteng;
[0085] The raw materials of agent A and agent B were crushed respectively,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com