Ornidazole injection and preparation method thereof
A technology of ornidazole injection and ornidazole, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of poor transportation stability, insoluble particles exceeding the standard, etc., and achieve the effect of good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
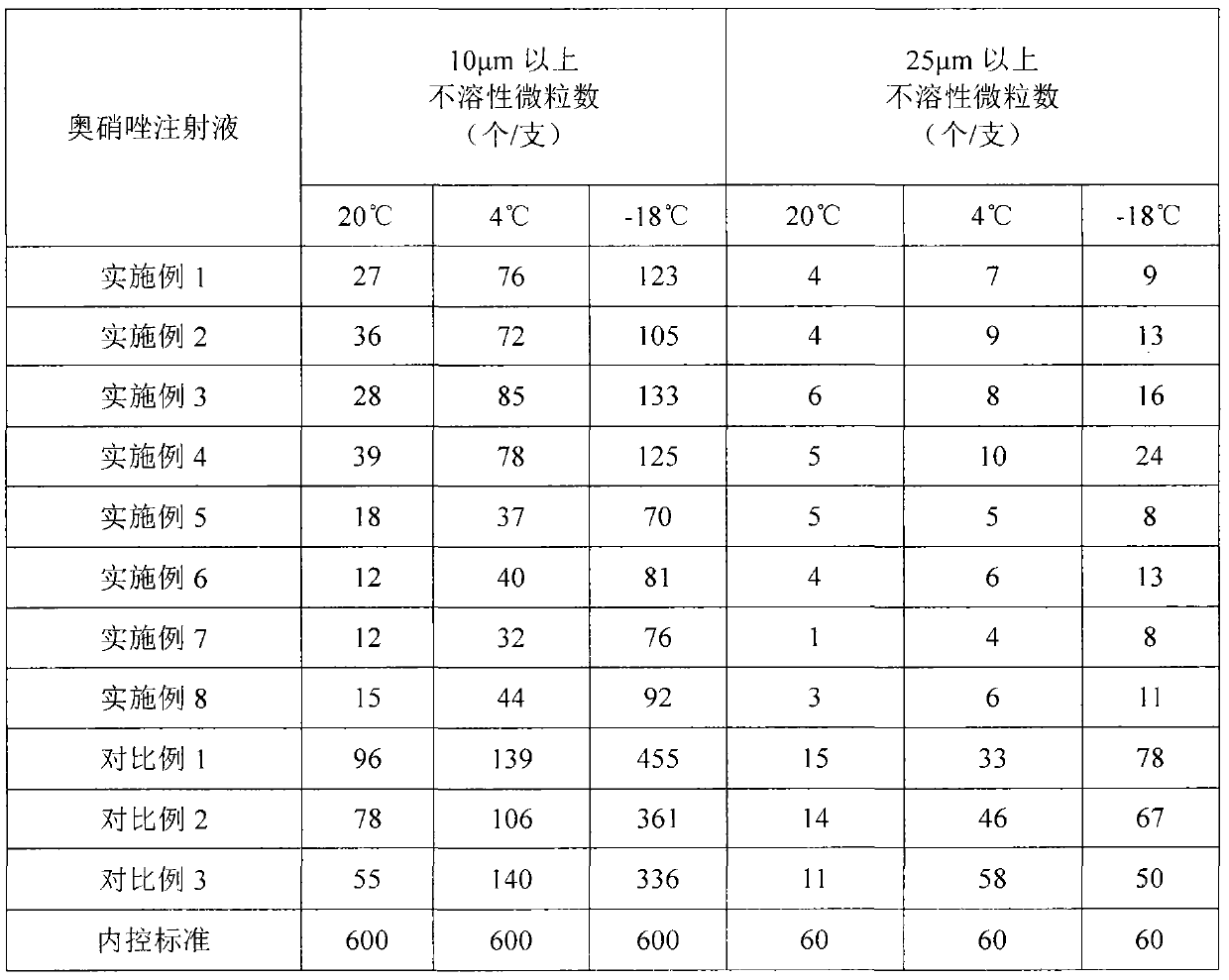
Examples
Embodiment 1
[0044] Ornidazole 10g
[0045] Propylene glycol 15g
[0047] Preparation:
[0048] (1) Mix ornidazole with propylene glycol and absolute ethanol, and the temperature of the mixed materials is 20°C;
[0049] (2) The uniformly mixed materials are heated to 50°C for 15 minutes, maintained at 50°C for 5 minutes, and then cooled to 20°C for 15 minutes to complete the first heating and cooling cycle. The material that has completed the first heating and cooling cycle is heated to 50°C in 15 minutes, maintained at 50°C for 5 minutes, and then cooled to 20°C in 15 minutes, completing the second heating and cooling cycle.
[0050] (3) Filter the material after two heating and cooling cycles with a filter membrane with a pore size of 0.22 μm, and potting, with a specification of 5 ml, to prepare the ornidazole injection of this comparative example.
Embodiment 2
[0052] prescription:
[0053] Ornidazole 10g
[0054] Propylene glycol 15g
[0055] Anhydrous ethanol 25g
[0056] Preparation:
[0057] (1) Mix ornidazole with propylene glycol and absolute ethanol, and the temperature of the mixed materials is 20°C;
[0058] (2) The uniformly mixed materials are heated to 40°C for 20 minutes, maintained at 40°C for 10 minutes, and then cooled to 25°C for 20 minutes to complete the first heating and cooling cycle. The material that has completed the first heating and cooling cycle is heated to 40°C in 20 minutes, maintained at 40°C for 10 minutes, and then cooled to 18°C in 20 minutes, completing the second heating and cooling cycle.
[0059] (3) Filter the material after two heating and cooling cycles with a filter membrane with a pore size of 0.22 μm, and potting, with a specification of 5 ml, to prepare the ornidazole injection of this comparative example.
Embodiment 3
[0061] prescription:
[0062] Ornidazole 10g
[0063] Propylene glycol 12.5g
[0064] Anhydrous ethanol 25g
[0065] Preparation:
[0066] (1) Mix ornidazole with propylene glycol and absolute ethanol, and the temperature of the mixed materials is 20°C;
[0067] (2) The uniformly mixed materials are heated to 45°C for 20 minutes, maintained at 45°C for 10 minutes, and then cooled to 23°C for 20 minutes to complete the first heating and cooling cycle. The material that has completed the first heating and cooling cycle is heated to 45°C in 20 minutes, maintained at 45°C for 10 minutes, and then cooled to 19°C in 20 minutes, completing the second heating and cooling cycle.
[0068] (3) Filter the material after two heating and cooling cycles with a filter membrane with a pore size of 0.22 μm, and potting, with a specification of 5 ml, to prepare the ornidazole injection of this comparative example.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com