A method for producing ticagrelor chiral pharmaceutical intermediates using Candida antarctica lipase b
A technology of Candida Antarctica and ticagrelor, applied in the field of enzyme engineering, can solve the problems of large amount of CALB enzyme and low purity of acetylated products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This example is the synthesis of the racemic ticagrelor drug intermediate with Cbz protecting group by organic chemistry; and the application of CALB in the resolution of the racemic drug intermediate with Cbz protecting group.
[0037] The CALB can be obtained by the following method, and its amino acid sequence is (GenBank accession No.Z30645.1);
[0038] A. Gene cloning and expression; references: Sun Jinpeng et al. (Study on the expression and enzymatic properties of Candida antarctica lipase B in Pichia pastoris, 2011).
[0039] B. The commercialized Candida antarctica lipase B was purchased from the market.
[0040] The method for synthesizing several classes of racemic ticagrelor pharmaceutical intermediates by the organic chemical method is as follows:
[0041] A. Synthesis steps of N-Benoxycarbonyl Hydroxylamine: Dissolve hydroxylamine hydrochloride (1.5mmol) and anhydrous potassium carbonate (0.75mmol) in a mixed solvent of ether: water 1:1, stir for about 1h...
Embodiment 2
[0057] The present embodiment is a preferred scheme based on the method in Example 1, and the improvements are as follows:
[0058] In the application of the CALB in racemic ticagrelor pharmaceutical intermediates:
[0059] Take CALB, add 1ml pH5 universal buffer, the enzyme concentration is preferably 0.08mg / ml, cancel the racemic ticagrelor intermediate and suspend evenly in the reaction solution, the substrate concentration is preferably 20g / L, wherein:
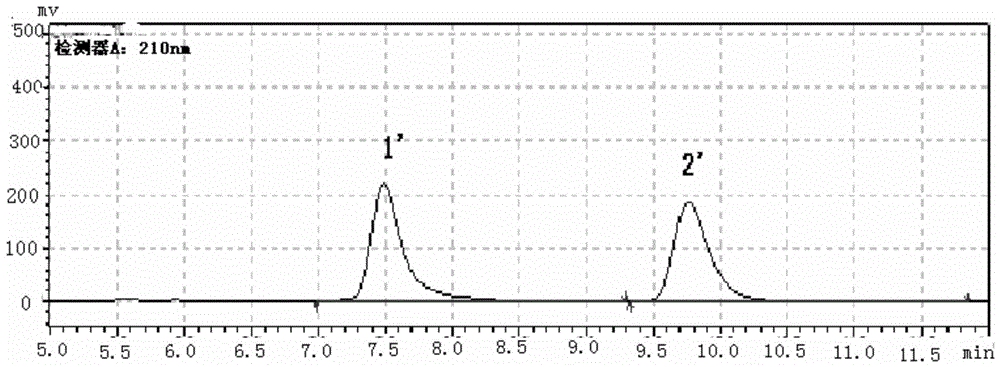
[0060] (1) The reaction temperature of cis-1-propionyloxy-N-benzyloxycarbonyl-4 amino-2-cyclopentene is preferably 60°C, and the reaction time is 8h. content, the obtained substrate resolution ee value is above 95%, the product (1S,4R) N-benzyloxycarbonyl-4-amino-2-cyclopenten-1-ol ee value can reach 99%, and the conversion rate is 45% %above.
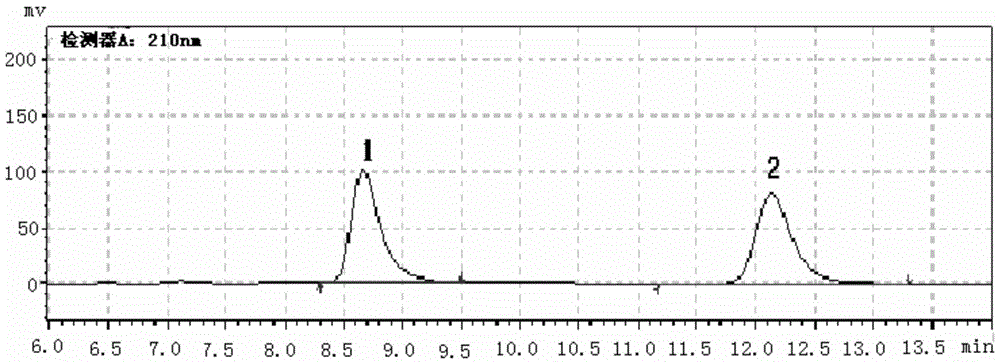
[0061] figure 1 It is the HPLC chiral spectrum of racemic cis-1-propionyloxy-N-benzyloxycarbonyl-4 amino-2-cyclopentene, as a blank chiral HPLC spectrum; figure 1 Among them, p...
Embodiment 3
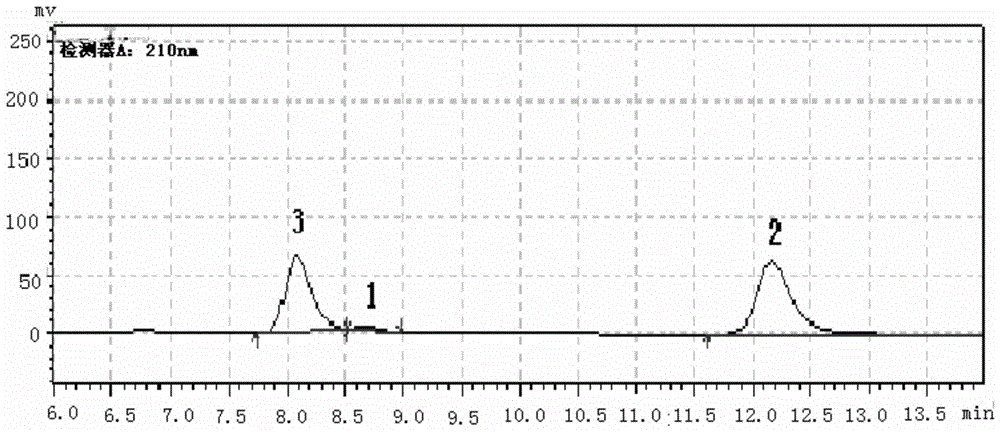
[0070] This example is the synthesis of cis-1-acetoxy-N-tert-butoxycarbonyl-4-amino-2-cyclopentene with a Boc protecting group by organic synthesis; and the use of CALB to replace the racemate Resolution studies performed on intermediate compounds of cagrel.
[0071] Described cis-1-acetoxy group-N-tert-butoxycarbonyl-4-amino-2-cyclopentene synthesis method is similar to the method described in Example 1, and the chloroformic acid in the A step in Example 1 is The ester was replaced by di-tert-butyl dicarbonate (Boc 2 O), and the rest of the steps are the same.
[0072] The application of the CALB in cis-1-acetoxy-N-tert-butoxycarbonyl-4-amino-2-cyclopentene is as follows:
[0073] Take CALB, add 1ml pH5 universal buffer, the enzyme concentration is about 0.08mg / ml, cancel the racemic ticagrelor intermediate and suspend evenly in the reaction solution, make the substrate concentration 20g / L, react at 60°C 4h, obtain reaction solution;
[0074] The reaction solution was ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com