Phthalazine or benzo phthalazine derivative and preparation method thereof
A technology of benzophthalazine and derivatives, applied in the field of phthalazine or benzophthalazine derivatives and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
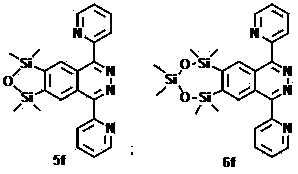
[0020]
[0021] 5a: Molecular formula: C 26 h 28 N 2 o 3 Si 2
[0022] Chinese name: 6,7-oxodisilyl-1,4-bis(4-methoxyphenyl)phthalazine
[0023] English name: 6,7-Oxadisilole-1,4-bis(4-methoxyphenyl) phthalazine
[0024] Molecular weight: 472.16
[0025] Appearance: yellow
[0026] H NMR (500 MHz, CDCl 3 ): δ 0.41 (s, 12H), 3.93 (s, 6H), 7.10-7.14 (m, 4H), 7.78 (d, J = 8.5 Hz, 4H), 8.31 (s, 2H) .
[0027] Carbon NMR (125 MHz, CDCl 3 ): δ 1.1, 55.5, 114.2, 125.7, 129.2, 131.6, 131.7, 152.0, 158.7, 160.7.
[0028] Maximum absorption wavelength: 323 nm (14.7×10 2 / M -1 cm) (CH 2 Cl 2 ).
[0029] Maximum emission wavelength: 482 nm (CH 2 Cl 2 ).
[0030] Quantum yield: 8.3% (CH 2 Cl 2 ).
[0031]
[0032] 6a: Molecular formula: C 28 h 34 N 2 o 4 Si 3
[0033] Chinese name: 6,7-Dioxytrisilyl-1,4-bis(4-methoxyphenyl)phthalazine
[0034] English name: 6,7-Dioxatrisilole-1,4-bis(4-methoxyphenyl) phthalazine
[0035] Molecular weight: 546.18
[0036]...
Embodiment 2
[0045]
[0046] 5b: Molecular formula: C 26 h 28 N 2 OSi 2
[0047] Chinese name: 6,7-oxodisilyl-1,4-bis(4-methylphenyl)phthalazine
[0048] English name: 6,7-Oxadisilole-1,4-bis(4-methylphenyl) phthalazine
[0049] Molecular weight: 440.17
[0050] Appearance: yellow
[0051] H NMR (500 MHz, CDCl 3 ): δ 0.42 (s, 12H), 2.51 (s, 6H), 7.43 (d, J = 8.0 Hz, 4H), 7.75 (d, J = 8.0 Hz, 4H), 8.33 (s, 2H) .
[0052] Carbon NMR (125 MHz, CDCl 3 ): δ 1.0, 21.5, 125.6, 129.1, 129.4, 130.2, 133.8, 139.3, 152.0, 159.2.
[0053] Maximum absorption wavelength: 309 nm (9.7×10 2 / M -1 cm) (CH 2 Cl 2 ).
[0054] Maximum emission wavelength: 435 nm (CH 2 Cl 2 ).
[0055] Quantum yield: 5.7% (CH 2 Cl 2 ).
[0056] 6b: Molecular formula: C 28 h 34 N 2 o 2 Si 3
[0057] Chinese name: 6,7-Dioxytrisilyl-1,4-bis(4-methylphenyl)phthalazine
[0058] English name: 6,7-Dioxatrisilole-1,4-bis(4-methylphenyl) phthalazine
[0059] 4-methylphenyl
[0060] Molecular weight: 5...
Embodiment 3
[0070]
[0071] 5c: Molecular formula: C 24 h 24 N 2 OSi 2
[0072] Chinese name: 6,7-oxodisilyl-1,4-diphenylphthalazine
[0073] English name: 6,7-Oxadisilole-1,4-diphenyl phthalazine
[0074] Molecular weight: 412.14
[0075] Appearance: yellow
[0076] H NMR (500 MHz, CDCl 3 ): δ 0.41 (s, 12H), 7.59-7.64 (m, 6H), 7.84-7.85 (m, 4H), 8.31 (s, 2H).
[0077] Carbon NMR (125 MHz, CDCl 3 ): δ 1.0, 125.6, 128.7, 129.1, 129.4, 130.3, 136.6, 152.3, 159.4.
[0078] Maximum absorption wavelength: 298 nm (6.3×10 2 / M -1 cm) (CH 2 Cl 2 ).
[0079] Maximum emission wavelength: 345 nm (CH 2 Cl 2 ).
[0080] Quantum yield: 13.1% (CH 2 Cl 2 ).
[0081]
[0082] 6c: Molecular formula: C 26 h 30 N 2 o 2 Si 3
[0083] Chinese name: 6,7-Dioxytrisilyl-1,4-diphenylphthalazine
[0084] English name: 6,7-Dioxatrisilole-1,4-diphenyl phthalazine
[0085] Molecular weight: 486.16
[0086] Appearance: yellow
[0087] H NMR (500 MHz, CDCl 3 ): δ 0.14 (s, 6H), 0.41 (s,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com