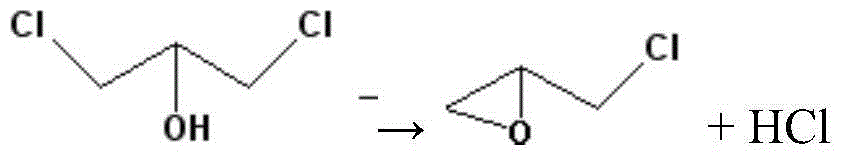
Method for removing gas phase hydrogen chloride from dichloropropanol to produce epichlorohydrin
A technology of epichlorohydrin and dichlorohydrin, applied in the directions of chlorine/hydrogen chloride, organic chemistry, etc., can solve the problems of unpublished methods for separating the oil phase of epichlorohydrin and solid catalyst, and achieves close interaction scale, Improve activity and stability, easy separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 10kg of calcium nitrate and 5kg of zirconium nitrate were mechanically stirred and mixed, sent to a high-energy ball mill for ball milling for 3 hours, and then sent to a catalyst roaster for 5 hours at 600°C to obtain an atom-mixed dichloropropanol cyclization catalyst. The high-energy ball mill made the catalyst Mixing at the atomic scale strengthens the interaction between metal cations, completely different from traditional impregnated supported catalysts, and improves the lifetime of cyclization catalysts.
[0025] The reaction uses a basic catalyst mixed with the above atoms, a gas-solid phase tube reactor, and the tube reactor is filled with the basic catalyst mixed with the above atoms, the reaction temperature is 160 ° C, the reaction pressure is 50KPaG, dichloropropanol catalytic In addition to hydrogen chloride to generate epichlorohydrin, after partial condensation, the crude epichlorohydrin is condensed as a product, and the gas phase hydrogen chloride is wa...
Embodiment 2
[0028] 22kg of calcium nitrate and 1kg of sodium nitrate were mechanically stirred and mixed, sent to a high-energy ball mill for ball milling for 20 hours, and then sent to a catalyst roaster for 5 hours at 500°C to obtain an atom-mixed dichloropropanol cyclization catalyst. The high-energy ball mill made the catalyst Mixing at the atomic scale strengthens the interaction between metal cations, completely different from traditional impregnated supported catalysts, and improves the lifetime of cyclization catalysts.
[0029] The reaction adopts the basic catalyst mixed with the above atoms, and the gas-solid phase column reactor is filled with the basic catalyst mixed with the above atoms in the fixed bed reactor. The reaction temperature is 60 ° C, the reaction pressure is 1KPaA, and the catalytic desorption In addition to hydrogen chloride to generate epichlorohydrin, after partial condensation, the crude epichlorohydrin is condensed as a product, and the gas phase hydrogen c...
Embodiment 3
[0032] 4kg of calcium nitrate and 2kg of zirconium nitrate were mechanically stirred and mixed, sent to a high-energy ball mill for ball milling for 3 hours, and then sent to a catalyst roaster for 5 hours at 600°C to obtain an atom-mixed dichloropropanol cyclization catalyst. The high-energy ball mill made the catalyst Mixing at the atomic scale strengthens the interaction between metal cations, completely different from traditional impregnated supported catalysts, and improves the lifetime of cyclization catalysts.
[0033]The reaction uses a basic catalyst mixed with the above atoms, a gas-solid phase reactor, and the fluidized bed reactor is filled with the basic catalyst mixed with the above atoms. The reaction temperature is 190 ° C, the reaction pressure is 50KPaG, dichloropropanol Remove hydrogen chloride to generate epichlorohydrin, after partial condensation, the crude epichlorohydrin is condensed as a product, the gas phase hydrogen chloride is washed and purified, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com