Synthetic process of bismuth-doped potassium niobate
A synthesis process, the technology of potassium niobate, which is applied in the field of synthesis process of bismuth-doped potassium niobate, can solve the problems of many impurities, uneven crystal form, expensive ethoxide, etc., and achieve lower reaction temperature, high sintering activity, good The effect of applying value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
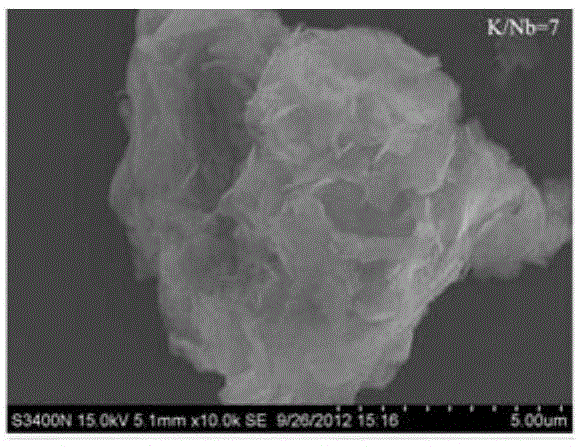
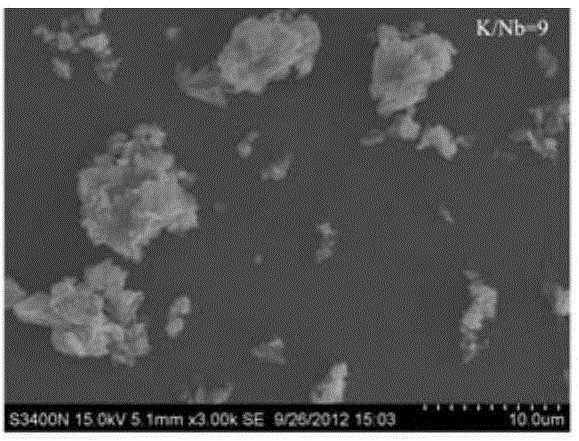
[0024] 0.2000g analytically pure Nb 2 o 5 Add 0.3604g of analytically pure KOH to 25ml of deionized water, stir until evenly mixed, then transfer to a hydrothermal kettle, tighten the lid of the kettle, and place it in an oven at 240°C for 24 hours to obtain monoclinic Structural K 4 Nb 6 o 17 niobate with a lattice constant of This complies with the standard card JCPDS76-0977. After the reaction, the reaction kettle was taken out, naturally cooled to room temperature, the lid of the kettle was opened, the product was taken out, filtered, washed several times with deionized water and absolute ethanol, and dried under normal pressure at 60°C to obtain potassium niobate.
Embodiment 2
[0026] 0.2000g analytically pure Nb 2 o 5 Add 0.7723g of analytically pure KOH to 25ml of deionized water, stir until evenly mixed, then transfer to a hydrothermal kettle, tighten the lid of the kettle, and place it in an oven at 240°C for 24 hours to obtain monoclinic Structural K 4 Nb 6 o 17 niobate with a lattice constant of This complies with the standard card JCPDS76-0977. After the reaction, the reaction kettle was taken out, naturally cooled to room temperature, the lid of the kettle was opened, the product was taken out, filtered, washed several times with deionized water and absolute ethanol, and dried under normal pressure at 60°C to obtain potassium niobate.
Embodiment 3
[0028] 0.2000g analytically pure Nb 2 o 5 Add 2.0594g of analytically pure KOH to 25ml of deionized water, stir until evenly mixed, then transfer to a hydrothermal kettle, tighten the lid of the kettle, and place it in an oven at 240°C for 24 hours to obtain monoclinic Structural K 4 Nb 6 o 17 niobate with a lattice constant of This complies with the standard card JCPDS76-0977. After the reaction, the reaction kettle was taken out, naturally cooled to room temperature, the lid of the kettle was opened, the product was taken out, filtered, washed several times with deionized water and absolute ethanol, and dried under normal pressure at 60°C to obtain potassium niobate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com