Paliperidone derivative slow release microsphere preparation and preparation method thereof
A slow-release microsphere preparation, the technology of paliperidone, is applied in the field of paliperidone derivative sustained-release microsphere preparation, additives and microsphere preparation, to achieve the effects of alleviating pain, reducing toxic and side effects, and low dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
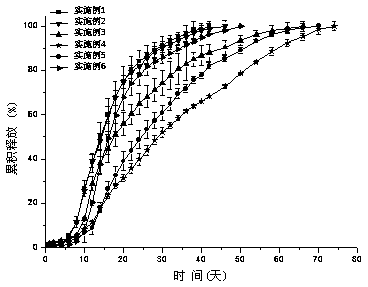
[0051] Weigh 0.8743 g 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl}-2-methyl-4-oxo Di-6,7,8,9-tetrahydro-pyrido[1,2-α]pyrimidin-9-yl isobutoxycarboxylate (see structural formula II) and 2.04 g 75257E PLGA, weigh the above Add to 13.60 ml of dichloromethane to dissolve, inject it into 1700ml of aqueous solution containing 0.5% PVA (w / w) under homogeneous (1200 - 2400rpm) conditions, keep the above homogeneous conditions for 5 minutes, and then The solvent was evaporated at 1200rpm for 4 hours, filtered through a sieve with a pore size of 25 μm and 125 μm, the microspheres were washed three times with distilled water, and freeze-dried. Microspheres containing 26% of the drug were prepared, the embedding rate was 87.56%, and the measured particle size was 1-200 μm. The line graph of the cumulative release rate of the sustained-release microspheres in the simulated release solution is shown in figure 1 ; The DSC experimental spectrum of sustained-release microsp...
Embodiment 2
[0053] Weigh 0.8743 g 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl}-2-methyl-4-oxo Generation-6,7,8,9-tetrahydro-pyrido[1,2-α]pyrimidin-9-yl isobutoxycarboxylate (see structural formula II), 2.04 g 75257E PLGA and 34.2 μl palmitic acid iso Propyl ester, add the above weighed substance into 13.60 ml of dichloromethane to dissolve, inject it into 1700ml of aqueous solution containing 0.5% PVA (w / w) under homogeneous conditions (1200 - 2400rpm), keep the above homogeneity Conditioned for 5 minutes, then the solvent was evaporated at a stirring speed of 1200 rpm for 4 hours, filtered through a sieve with a pore size of 25 μm and 125 μm, washed with distilled water three times, and freeze-dried. Microspheres containing 25.82% of the drug were prepared, the embedding rate was 86.05%, and the measured particle size was 1-200 μm. The line graph of the cumulative release rate of the sustained-release microspheres in the simulated release solution is shown in figure 1...
Embodiment 3
[0055] Weigh 0.8743 g 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl}-2-methyl-4-oxo Generation-6,7,8,9-tetrahydro-pyrido[1,2-α]pyrimidin-9-yl isobutoxycarboxylate (see structural formula II), 2.04 g 75257E PLGA and 68.2 μl palmitic acid iso Propyl ester, add the above weighed substance into 13.60 ml of dichloromethane to dissolve, inject it into 1700ml of aqueous solution containing 0.5% PVA (w / w) under homogeneous conditions (1200 - 2400rpm), keep the above homogeneity Conditioned for 5 minutes, then the solvent was evaporated at a stirring speed of 1200 rpm for 4 hours, filtered through a sieve with a pore size of 25 μm and 125 μm, washed with distilled water three times, and freeze-dried. Microspheres containing 26.79% of the drug were prepared, the embedding rate was 89.30%, and the measured particle size was 1-200 μm. The line graph of the cumulative release rate of the sustained-release microspheres in the simulated release solution is shown in figure 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com