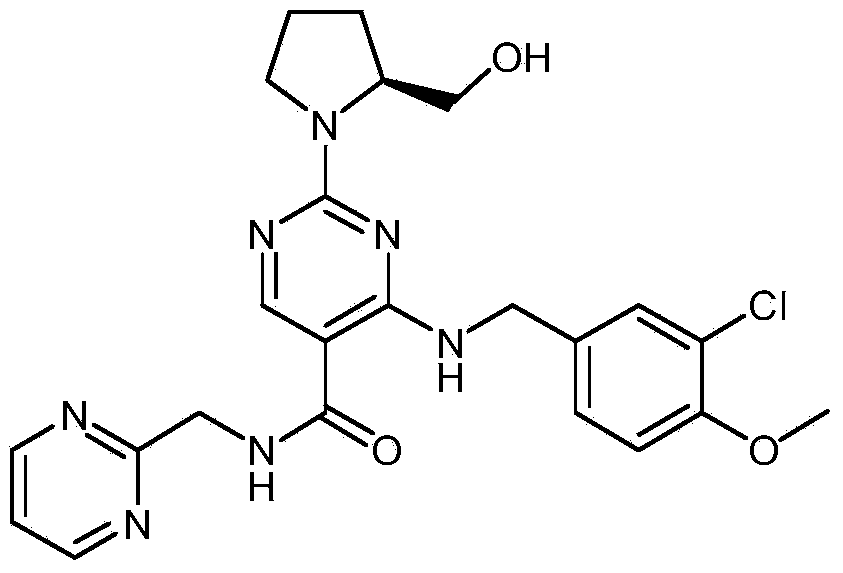
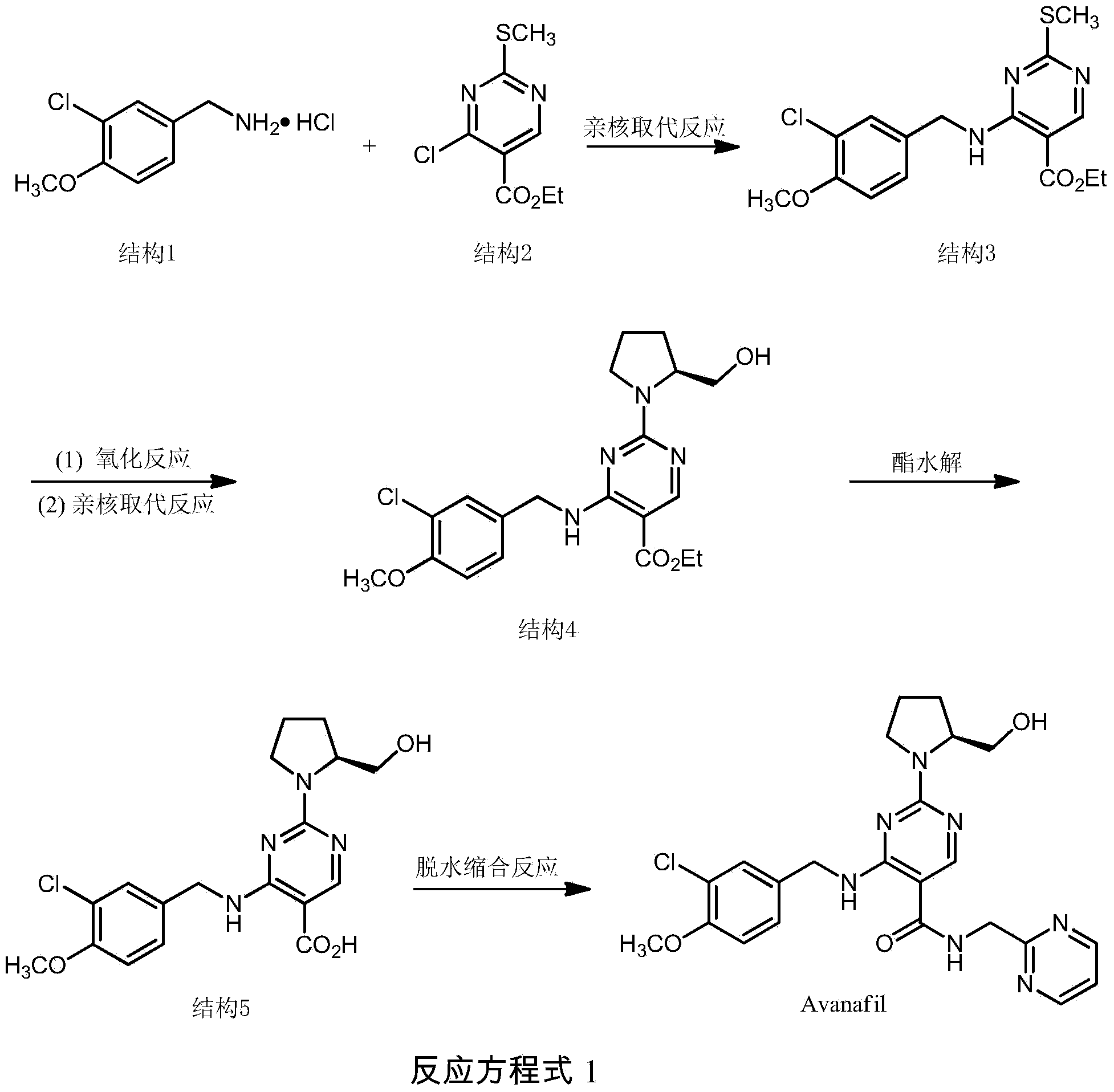
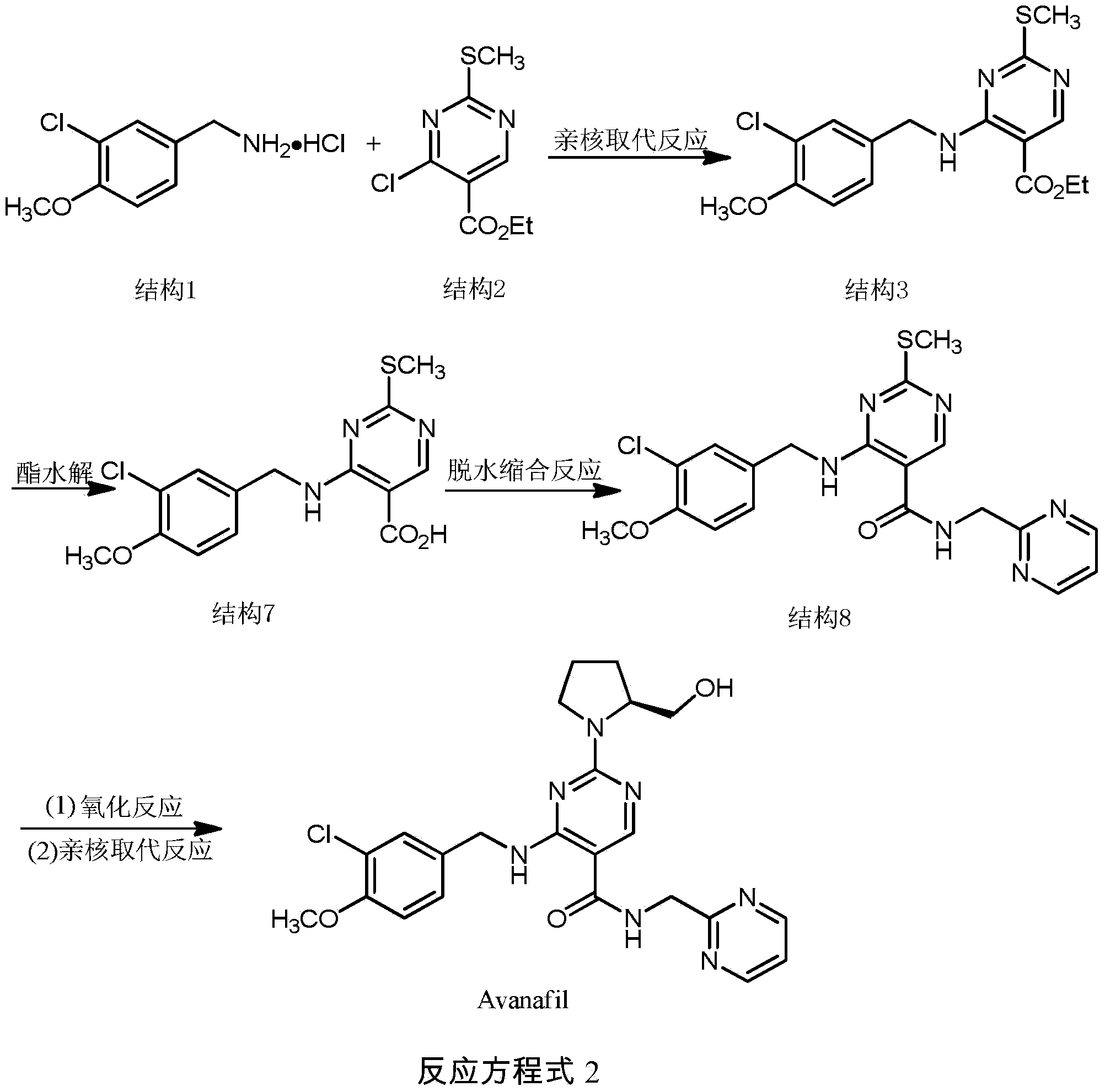
Synthesis method of avanafil
A technology of avanafil and synthetic method, which is applied in the field of organic chemical synthesis, can solve the problems of cumbersome operation, high cost, unfavorable industrial production, etc., and achieve the effect of high product purity, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of synthetic method of avanafil comprises the steps:
[0035] 1. Synthesis of 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (structure 3)
[0036] At room temperature, in a 50mL round bottom flask, add 3-chloro-p-methoxybenzylamine hydrochloride (0.68g, 3.3mol), N,N-dimethylformamide (4mL), triethylamine (1mL) , 4-Chloro-5-ethoxycarbonyl-2-methylthiopyrimidine (0.66 g, 2.8 mmol). Reaction at room temperature, electromagnetic stirring for 1 hour.
[0037] Crude product purification: add distilled water (15mL) to the crude product, extract with ethyl acetate (20mL×3), collect the organic layer and concentrate to 20mL, then add distilled water to wash (20mL), collect the organic phase, anhydrous Na 2 SO 4 After drying and rotary evaporation under reduced pressure, a white solid was obtained, which was recrystallized from ethyl acetate and petroleum ether, and finally 1.00 g of powdery white crystal was obtained, with a yield of 86% and...
Embodiment 2
[0052] A kind of synthetic method of avanafil comprises the steps:
[0053] 1. Synthesis of 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine (structure 3)
[0054] At room temperature, in a 100mL round bottom flask, add 3-chloro-p-methoxybenzylamine hydrochloride (2.00g, 9.70mol), N,N-dimethylformamide (6mL), triethylamine (6mL) , 4-Chloro-5-ethoxycarbonyl-2-methylthiopyrimidine (1.94 g, 8.24 mmol). Reaction at room temperature, electromagnetic stirring for 1 hour.
[0055] Crude product purification: add distilled water (20mL) to the crude product, extract with ethyl acetate (20mL×3), collect the organic layer and concentrate to 20mL, then add distilled water to wash (20mL), collect the organic phase, anhydrous Na 2 SO 4 After drying and rotary evaporation under reduced pressure, a white solid was obtained, which was recrystallized from ethyl acetate and petroleum ether, and finally 2.91 g of powdery white crystals were obtained, with a yield of 85...
Embodiment 3
[0069] A synthetic method of avanafil, comprising the following steps:
[0070] (1) Add 3-chloro-p-methoxybenzylamine hydrochloride, N,N-dimethylformamide, triethylamine, 4-chloro-5-ethoxycarbonyl-2-methylthiopyrimidine to the container Carry out nucleophilic substitution reaction to obtain 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine, the reaction temperature of the nucleophilic substitution reaction is 0-40 °C, the reaction time is 1-2 hours.
[0071] (2) Add m-CPBA, 4-(3-chloro-4-methoxybenzylamino)-5-ethoxycarbonyl-2-methylthiopyrimidine to obtain 4-(3-chloro-4 -methoxybenzylamino)-5-ethoxycarbonyl-2-methylsulfonylpyrimidine; the reaction temperature of the oxidation reaction is 10-40° C., and the reaction time is 1-3 hours.
[0072] (3) adding triethylamine, 4-(3-chloro-4-methoxybenzyl amino)-5-ethoxycarbonyl-2-methylsulfonyl pyrimidine and L-prolinol carry out nucleophilic reaction to obtain ( S)-4-(3-chloro-4-methoxybenzylamino)-5-ethoxyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com