New application of 5-HT3AR inhibitor Ondansetron
A 1.5-HT3AR, 2.5-HT3AR technology, applied in the field of 5-HT3AR inhibitor Ondansetron, can solve the problem of not being able to effectively simulate all symptoms of clinical pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
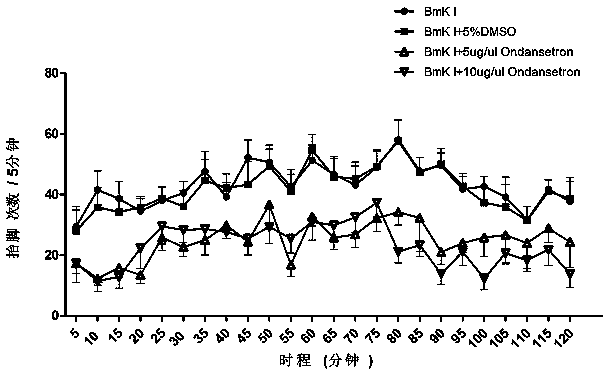
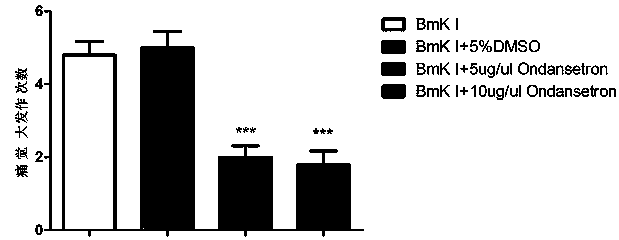
[0017] Example 1: Observation of Different Doses of Ondansetron Relieving Rats' Spontaneous Paw Withdrawal and Painful Grand Attack Response
[0018] Put a 75 cm high bracket on the table covered with a glass mirror, and place a 20×20×30cm 3 Transparent plexiglass box, from the mirror on the table to observe the reaction of rat feet after administration. Before the drug injection, the rats were placed in the box 30 minutes in advance to adapt to the experimental environment. After the plantar injection of BmK I, the animals were put back into the detection box, and the spontaneous pain behavior was continuously observed. The nociceptive spontaneous pain response was evaluated by the number of times of spontaneous foot lifting every 5 minutes, the number of screams and other great pain attacks. The way of administration of Ondansetron is to inject 10ul of 5ug / ul or 10ug / ul intrathecally into rats 30 minutes before BmK I injection. Ondansetron's relief of spontaneous pain a...
Embodiment 2
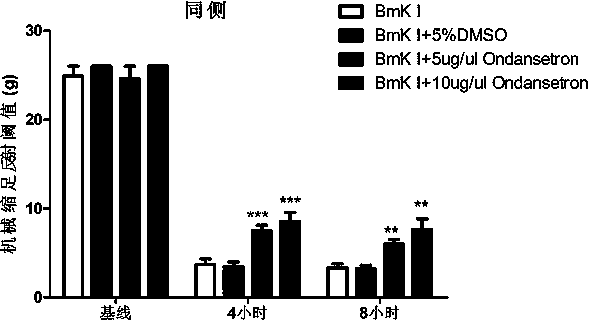
[0020] Example 2: Observation of Different Doses of Ondansetron Alleviating Bilateral Mechanical Hyperalgesia in Rats
example 1
[0021] After the spontaneous pain disappeared substantially in Example 1, the rats were placed on a steel wire grid (1cm 2 ) on a plexiglass box (20×20×30 cm 3 )middle. A series of Von Frey fibers (58011, Stoelting Co., USA) (from 0.6 to 26 g) were used to measure the mechanical withdrawal reflex threshold. It can be measured when the rat's injected hind foot touches the ground and bears normal weight. Stimulate the skin of the plantar center of the hindlimb of the animal on the injection side from under the grid, bend each fiber to an appropriate degree and stay for 2-3 seconds, and the interval between two measurements is 10 seconds. The stimulation intensity of Von Frey fibers ranged from small to large. If there is no withdrawal reflex, continue with a larger grammage of fiber, up to a maximum of 26 g. The minimum gram value that can cause 50% paw withdrawal response was taken as the mechanical paw withdrawal reflex threshold. The mechanical pain threshold was collect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com