Aromatic-cationic peptides and methods for using same
A subject and surgical technique, applied in the direction of peptides, tripeptide components, tetrapeptide components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0162] II. Preparation of Aromatic-Cationic Peptides of the Present Technology
[0163] The aromatic-cationic peptides of the present technology are water-soluble and highly polar. Despite these properties, the peptide can easily penetrate cell membranes. The aromatic-cationic peptide typically comprises a minimum of 3 amino acids or a minimum of 4 amino acids covalently linked by peptide bonds. The maximum number of amino acids present in the aromatic-cationic peptide is about 20 amino acids covalently linked by peptide bonds. Suitably, the maximum number of amino acids is about 12, more preferably about 9 and most preferably about 6.
[0164] The amino acid of the aromatic-cationic peptide can be any amino acid. The term "amino acid" as used herein is intended to refer to any organic molecule comprising at least one amino group and at least one carboxyl group. Typically, at least one amino group is alpha to the carboxyl group. The amino acid may be naturally occurring...
Embodiment 1
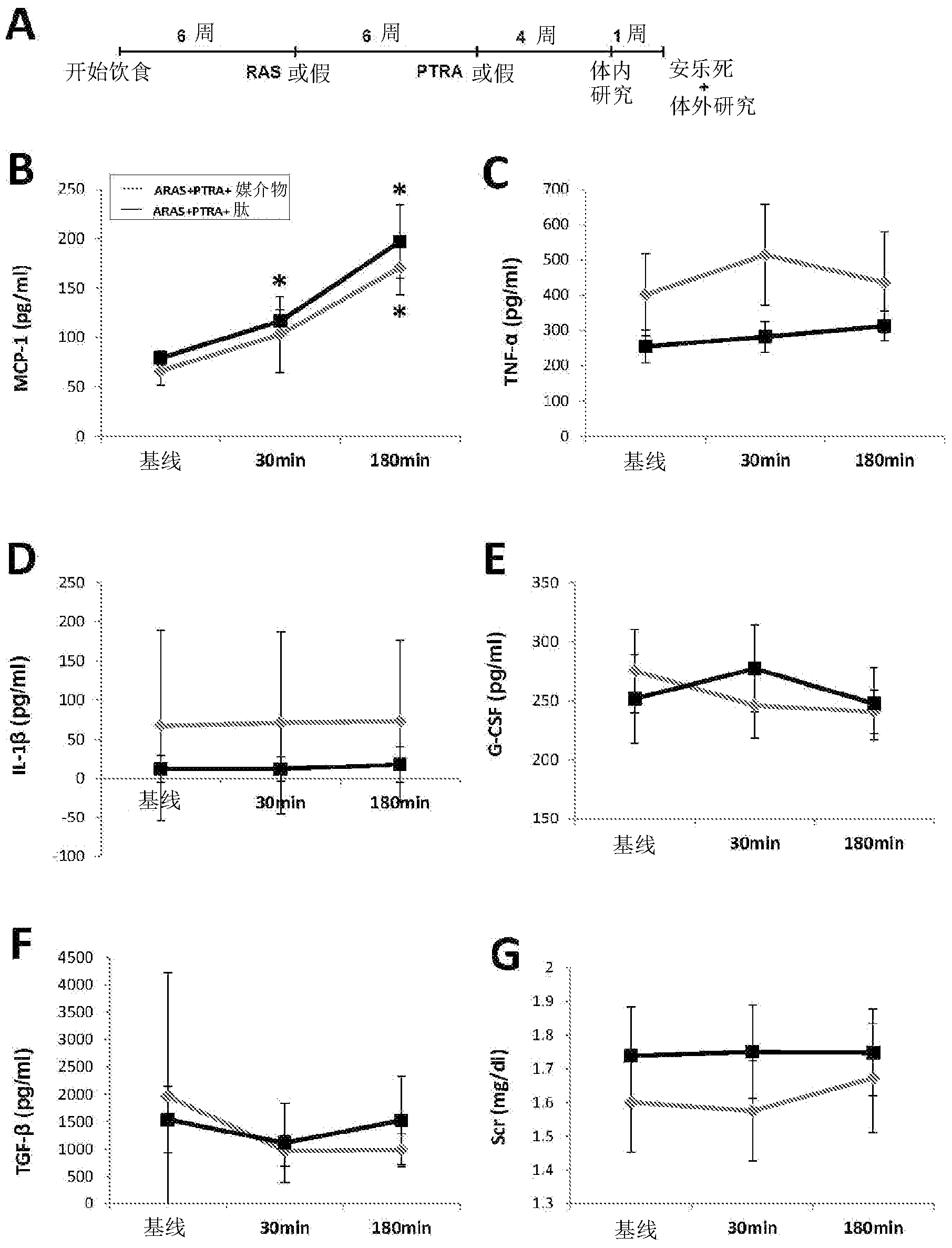
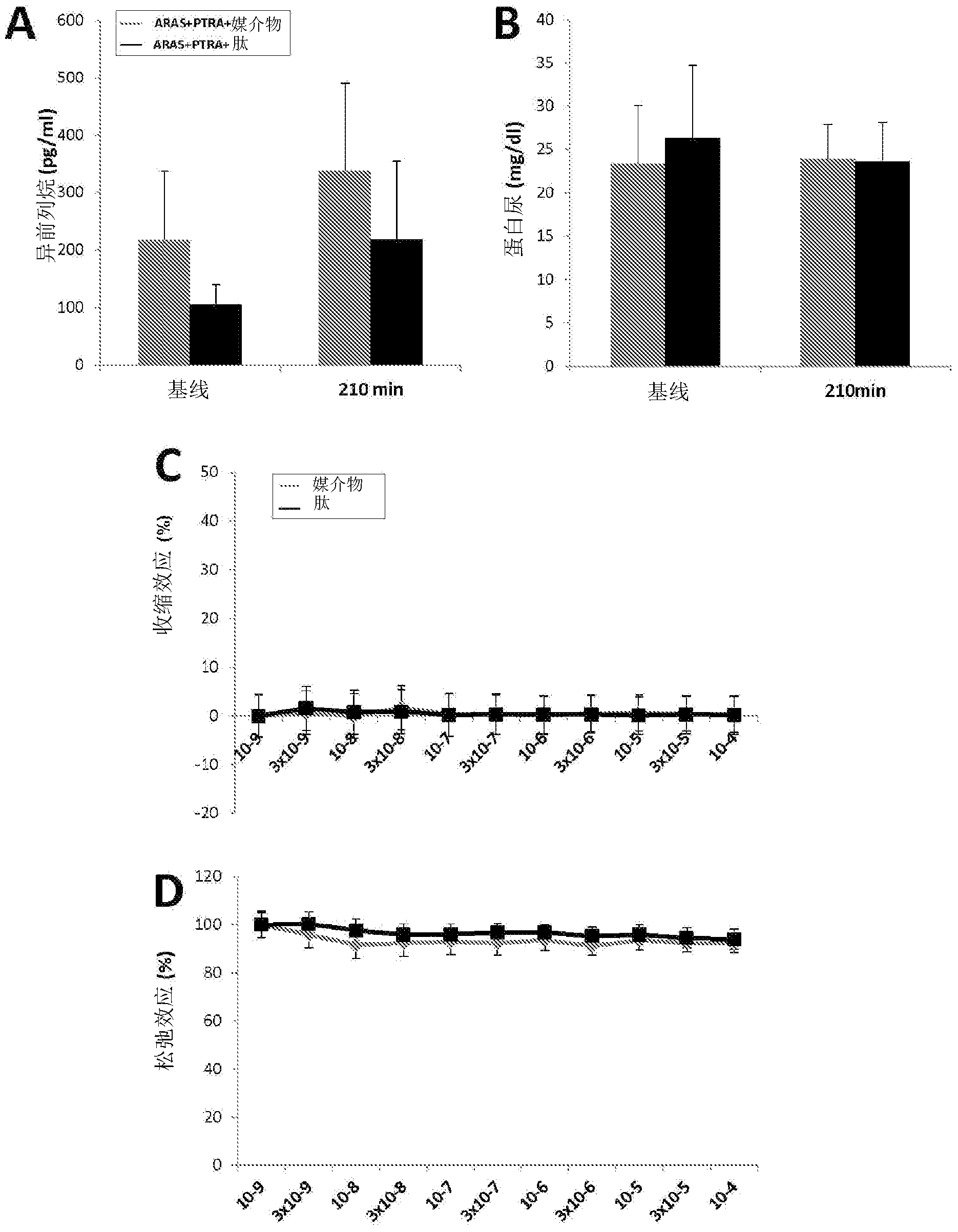
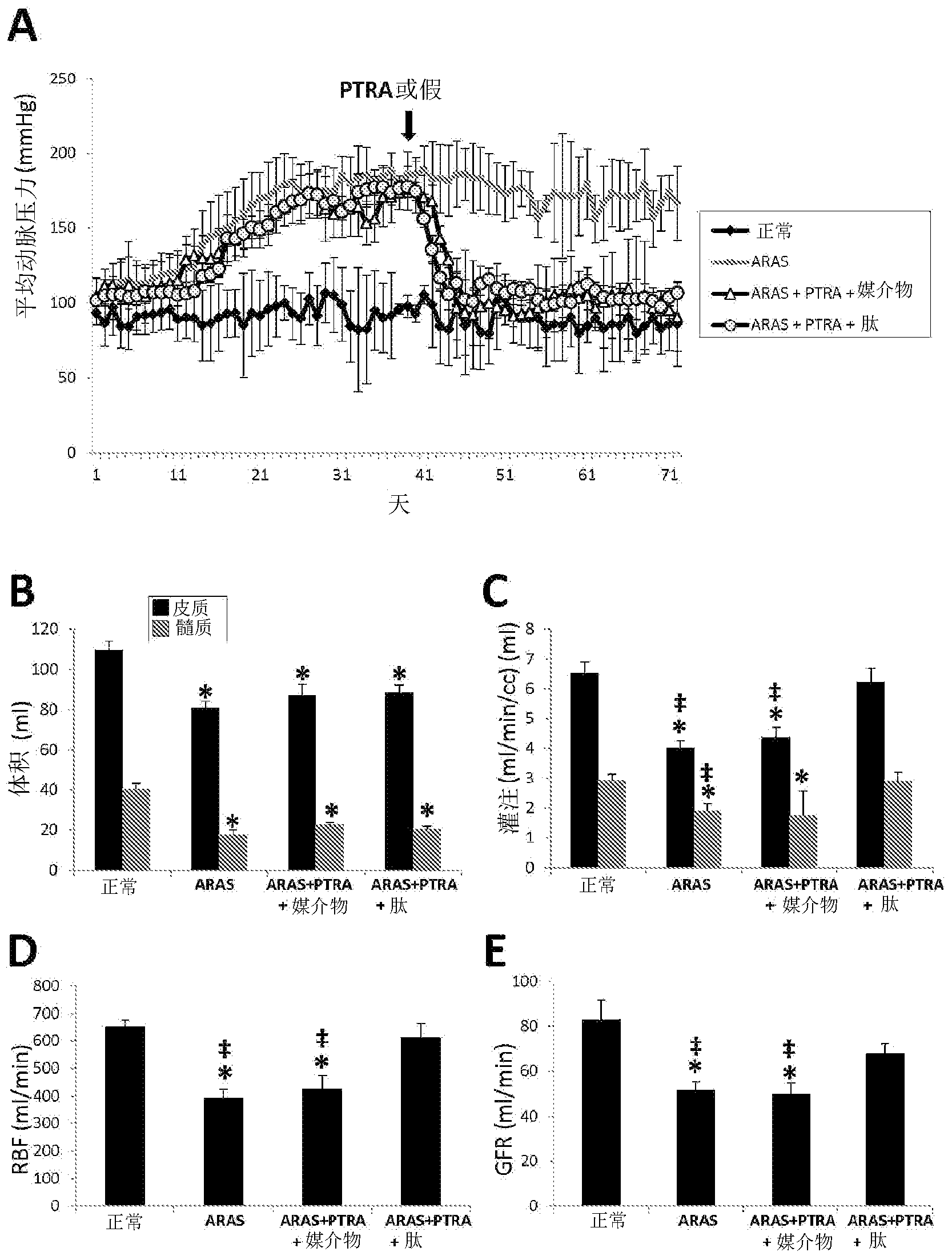
[0276] Example 1 by using D-Arg-2', 6'-Dmt-Lys-Phe-NH 2 to alleviate porcine renal atherosclerosis Renal damage after percutaneous transluminal renal angioplasty (PTRA) in arterial stenosis-like stenosis (ARAS) hurt.
[0277] A. Introduction
[0278] This example confirms that the aromatic cationic peptide D-Arg-2', 6'-Dmt-Lys-Phe-NH 2 Use in the prevention and treatment of renal injury associated with renal atherosclerotic arterial stenosis (ARAS). According to the methods of the invention, animal subjects are subjected to ARAS for a period of time, followed by renal revascularization by percutaneous transluminal renal angioplasty (PTRA). The aromatic-cationic peptide is administered to the subject in conjunction with PTRA, including for defined periods of time before and after revascularization surgery. Control animals received either an infusion or an infusion of control vehicle alone. Various aspects of renal function were improved in subjects receiving the p...
Embodiment 2
[0355] Example 2 by using D-Arg-2', 6'-Dmt-Lys-Phe-NH 2 to alleviate atherosclerosis in humans Renal damage after percutaneous transluminal renal angioplasty (ARAS) in sclerotic renal artery stenosis (ARAS) hurt
[0356] A. Overview
[0357] This example will confirm that the aromatic cationic peptide D-Arg-2', 6'-Dmt-Lys-Phe-NH 2 Use in the prevention and treatment of renal injury associated with renal atherosclerotic artery stenosis (ARAS) in humans. According to the methods of the invention, a human subject with ARAS undergoes renal revascularization by percutaneous transluminal renal angioplasty (PTRA). The subject is administered the aromatic-cationic peptide conjugated to PTRA, including for defined periods of time before and after revascularization surgery. Control subjects either received no infusion or received an infusion of control vehicle alone. Multiple aspects of renal function are expected to be improved in subjects receiving the peptide (compared ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com