ELISA kit for severe patient disease evaluation and detection method thereof
A detection method and kit technology, applied in the field of immunoassay medicine, can solve the problems of low accuracy, long cycle, complicated operation, etc., and achieve good specificity and sensitivity, accurate disease severity assessment, simple and rapid early assessment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
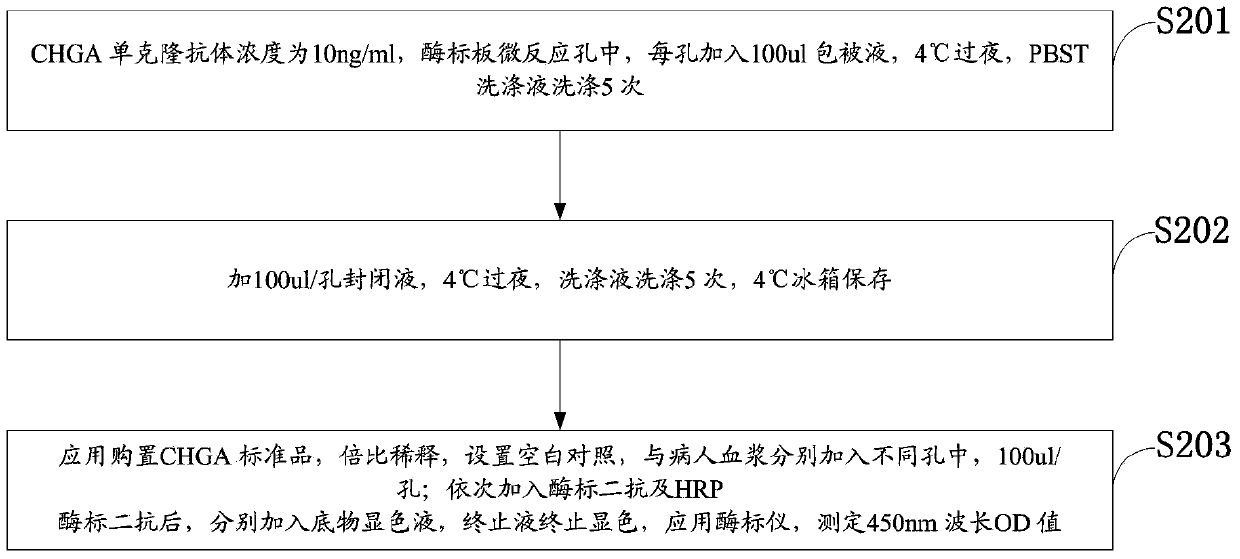
[0028] Experimental example 1, preparation of ELISA kit and detection method for condition assessment of critically ill patients
[0029] 1.1 Monoclonal antibody: use antibodies CHGA monoclonal antibody, product number ABIN933034, specification 100ul (1mg / ml), antibody type LGG1kappa;
[0030] 1.2 Coating: Coating formula, the concentration of CHGA monoclonal antibody is 10ng / ml, add 100ul coating solution to each well of the micro-reaction plate of the microplate, overnight at 4°C, wash 5 times with PBST washing solution;
[0031] 1.3 Blocking: add 100ul / well blocking solution, overnight at 4°C, wash with washing solution 5 times, store in refrigerator at 4°C;
[0032] 1.4 Measurement: Use the purchased CHGA standard product, double dilution, set up a blank control, and add the patient's plasma into different wells (100ul / well); after adding the secondary antibody and HRP enzyme-labeled secondary antibody in sequence, add the substrate chromogenic solution respectively , sto...
Embodiment 2
[0033] Example 2, a study on the correlation between the severity of illness and plasma CHGA concentration in critically ill patients.
[0034] 2.1 Methods: Using the above ELISA method, 20 healthy volunteers and 120 consecutive critically ill patients were tested for plasma CHGA within 24 hours after admission, PCT, CRP and other indicators were detected, and APACHEII and SOFA scores and 28-day mortality were recorded.
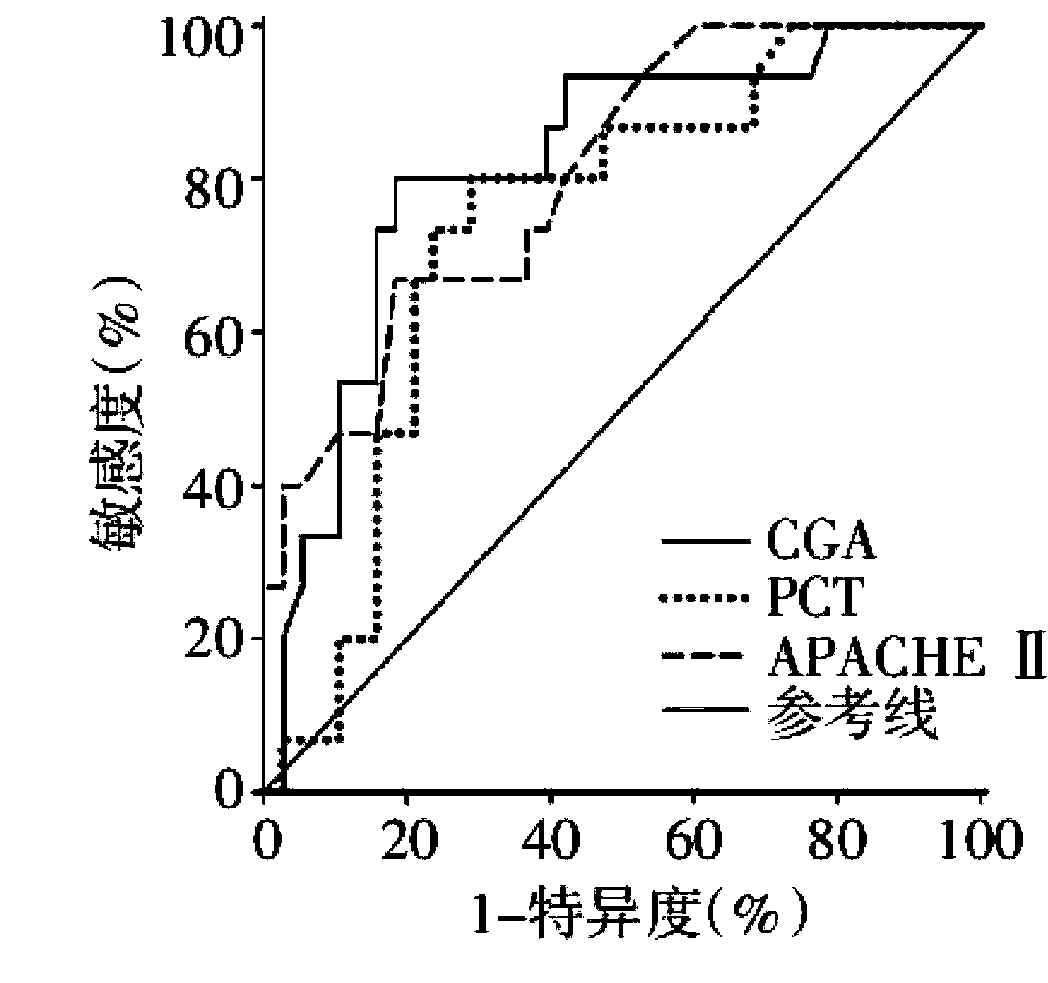
[0035] 2.2 Results: The plasma CHGA level of the healthy control group: 40.91±4.63ug / L, the plasma CHGA level of the septic shock group: 213.62±36.90ug / L, the CHGA concentration of the dead patients was significantly higher than that of the surviving patients [(190.51±19.26ug / L) vs(65.00±16.87ug / L)p<0.017], ROC analysis takes 139ug / L as the cutoff value, the sensitivity of CHGA to predict death is 80.0%, and the specificity is 81.6%.
[0036] Such as image 3 As shown, the results of ROC analysis show that: CHGA and APACHEII score have comparable ability to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com