A mutant of alkaline pectinase with improved thermostability
A thermostability and pectinase technology, applied in the field of bioengineering, can solve the problems of commercial alkaline pectinase, which cannot be ignored in research, and achieve the effect of enhanced thermostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The construction of embodiment 1 mutant expression plasmid and the acquisition of recombinant Bacillus subtilis
[0025] 1. Construct a mutant expression vector using the pET-20b(+)-pgl plasmid as a template
[0026] The nucleotide sequence of the gene encoding the wild-type alkaline pectinase and the signal peptide consisting of 21 amino acids is shown in SEQ ID NO.1, and the amino acid sequence of the wild-type mature alkaline pectinase is shown in SEQ ID NO.2. By comparing the heat-resistant pectinase sequences: CAD56882 of Bacilluslicheniformis, BAA96478 from Bacillussp.strainP-4-N, and AAD35518 from Thermotogamaritima MSB82, the optimum temperatures were 69°C, 70°C and 90°C, respectively. Combined with the three-dimensional structure of alkaline pectinase, it is speculated that the serine S at position 229 has a greater impact on the thermal stability of alkaline pectinase, and a mutation experiment was designed to mutate the serine S at position 229 into the other...
Embodiment 2
[0039] Expression of embodiment 2 mutant PGL
[0040] Seed medium composition (g / L): yeast powder 5, tryptone 10, NaCl 10, glucose 20, pH 7.0.
[0041] Composition of fermentation medium: yeast powder 24g / L, tryptone 12g / L, glycerol 5g / L, K 2 HPO 4 72mmolL -1 , KH 2 PO 4 17mmolL -1 .
[0042] Inoculate the recombinant bacteria E.coliBL21(DE3) containing the mutant expression vector pET-20b(+)-pglS229K from a glycerol tube into 100μgmL -1 In the seed medium of ampicillin, the filling volume is 20mL / 250mL. The culture temperature was 37° C., and the culture was shaken on a shaker at 200 rpm for 10 h.
[0043] The seed solution cultivated for 10h was inoculated with 100μgmL with a 3% (V / V) inoculum -1 In the fermentation medium of ampicillin, the filling volume is 50mL / 500mL, at 37°C, 200rmin -1 to cultivate. Bacteria grow to a certain stage (OD 600 =0.6), adding a final concentration of 0.4mMIPTG for induction, while adjusting the temperature to 30°C, and inducing fe...
Embodiment 3
[0044] Enzymatic properties of PGL before and after mutation in embodiment 3
[0045] According to the method described in Example 2, the E.coliBL21(DE3) containing the unmutated expression vector pET-20b(+)-pgl and the mutant strain E.coliBL21(DE3)(pET-20b(+)-pglS229K ) is fermented, and the enzymes in the fermentation broth are purified and analyzed for enzymatic properties such as heat resistance. The resulting alkaline pectinase mutant was named S229K.
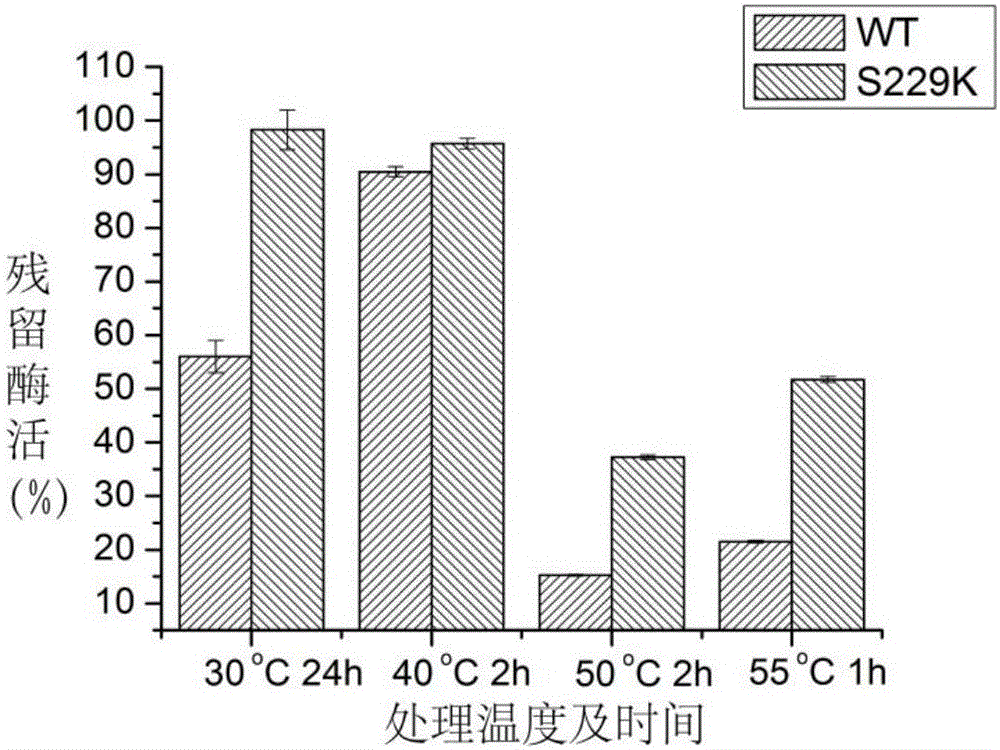
[0046] Depend on figure 1 It can be seen that the enzyme activity loss of alkaline pectinase (WT) before mutation is obvious after incubation at 30°C for 24h, 50°C for 2h, and 55°C for 1h; but after mutation, alkaline pectinase (S229K) After doing the same treatment, it still maintains a high residual enzyme activity. After incubation at 30°C for 24 hours, the residual enzyme activity after mutation increased by 42.29% compared with that before mutation; after incubation at 50°C for 2 hours, the residual enzyme activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com