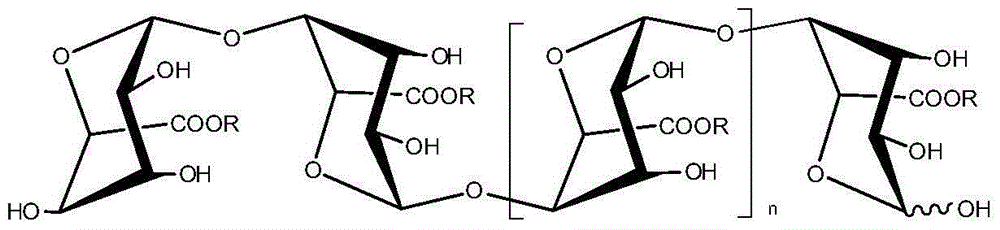
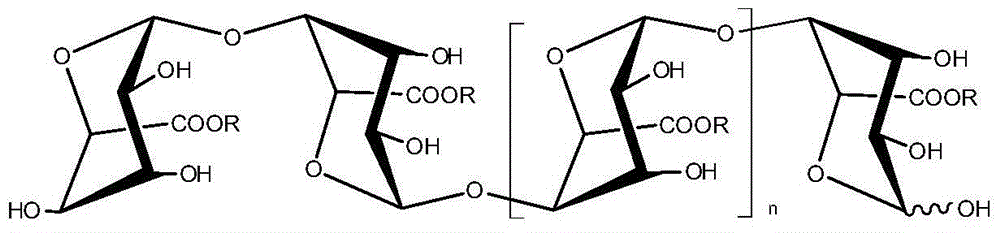
Application of oligomeric guluronic acid salts in preparation of Parkinson's disease prevention and treatment drugs or products
A technology of guluronic acid salt and polyguluronic acid, applied in the field of marine medicine, can solve problems such as anti-Parkinson's disease that have not yet been seen, and achieve broad development and application prospects, abundant resources, and easy industrialization. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of oligomeric guluronic acid salt (LPG)
[0025] Prepare alginate (sodium alginate in this example) into a 10wt% aqueous solution, heat it to 80-90°C with 1wt% dilute hydrochloric acid, stir and degrade it for 4-5 hours, neutralize it with 10wt% sodium carbonate aqueous solution after cooling, and then Use 5wt% dilute hydrochloric acid to adjust the pH to about 3.65, centrifuge to collect the precipitate, dissolve it with 2mol / L NaOH, add 3 times the volume of 95wt% ethanol, collect the precipitate, dehydrate with absolute ethanol and dry to obtain polyguluronic acid sodium salt (Mw=11.2kD). The polyguluronic acid sodium salt was formulated with pure water into 10wt% aqueous solutions of different concentrations, and degraded by the Fenton method to obtain oligomeric guluronic acids with different molecular weights. After the oligomeric guluronic acid is neutralized by lithium hydroxide, sodium hydroxide, potassium hydroxide, calcium hydroxide...
Embodiment 2
[0030] Embodiment 2: the present invention adopts MPP + Inducing SK-N-MC neuroblasts to establish a Parkinson's disease model. The model has clinical and pathological features similar to Parkinson's disease: the activity of mitochondrial complex I is significantly reduced, ATP energy deficiency, and mitochondrial dysfunction.
[0031] The present embodiment adopts the oligomeric guluronic acid sodium salt obtained in Example 1. When SK-N-MC cells adhered to the wall at about 80%, they were digested and passaged with 0.25wt% trypsin. SK-N-MC cells that grew well and were in the logarithmic phase were made into cell suspension, and then press 5×10 4 / mL seeded in 96-well cell culture plate, at 37°C, 5% CO 2 Cultivate in the cell incubator for 24 hours, suck away the old medium, and set up the blank control group, model group, positive control group (LA), and sample protection group (LPG) respectively. The LA concentration of the positive control group is 50 μM, and the sample...
Embodiment 3
[0038] The present embodiment adopts the oligomeric guluronic acid sodium salt obtained in Example 1. SK-N-MC cells that grew well and were in the logarithmic phase were made into cell suspension, and then press 5×10 4 / mL seeded in 96-well cell culture plate, at 37°C, 5% CO 2 Cultivate in the cell incubator for 24 hours, suck away the old medium, and set up the blank control group, model group, positive control group (LA), and sample protection group (LPG) respectively. The LA concentration of the positive control group is 50 μM, and the sample protection group Add 200 μL of fresh medium containing samples to make the final concentration of the drug 50 μM, respectively add medium containing equal volumes of ultrapure water to the blank control group and model group, and continue to cultivate for 24 hours. The cells were washed once with PBS, and the intracellular ROS level was detected with DCFH-DA fluorescent probe. DCFH-DA itself is a non-fluorescent substance. After ente...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com