A preparation method for controlling the valence state of tio2 nanotube loaded metal
A technology for supporting metals and nanotubes, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. Complex problems, to achieve the effect of not easy to damage, high experiment repetition rate, simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
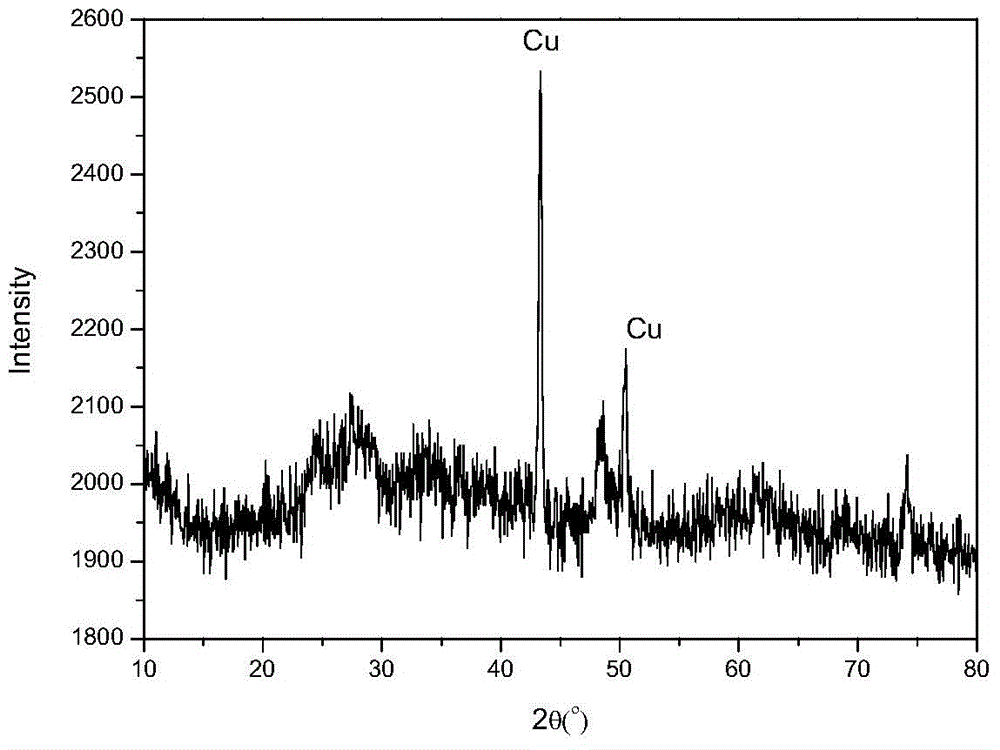
[0022] Example 1 Cu loaded iO 2 Preparation of nanotubes
[0023] (1) 0.8g TiO 2 (P25) with 100mL 10mol L -1 Mix KOH solution, stir for 30min, transfer to 150mL hydrothermal kettle with Teflon liner, and react hydrothermally at 150℃ for 24 hours;
[0024] (2) Cool to room temperature, pour off the supernatant, centrifuge to obtain a solid, wash three times with 100mL absolute ethanol, and centrifuge to obtain a solid;
[0025] (3) Control the mol ratio of glucose and copper acetate at 3 / 8, add in the ethanol solution of 0.02g morphology regulator polyethylene glycol, stir and pour into the hydrothermal kettle with polytetrafluoroethylene lining at 130 ℃ for 24 hours;
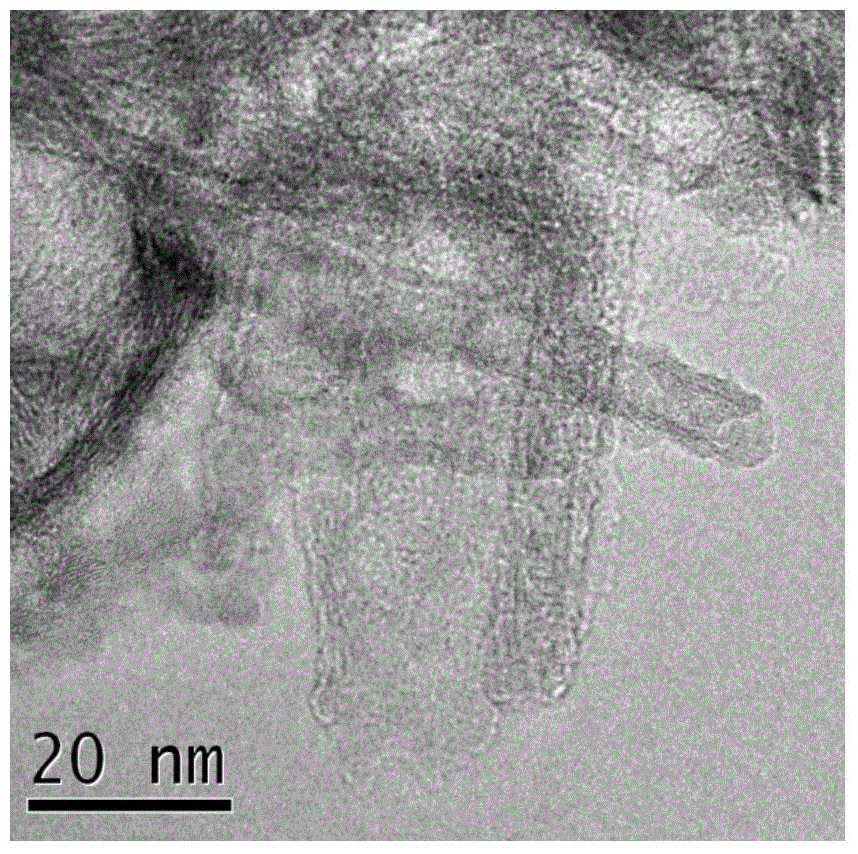
[0026] (4) Cool naturally, then wash off the organic matter and sodium ions on the surface with absolute ethanol and water. At this time, the pH value is about 7. Finally, the solid is vacuum-dried at 80°C for 5 hours and ground to obtain Cu-loaded TiO 2 nanotube.
Embodiment 2
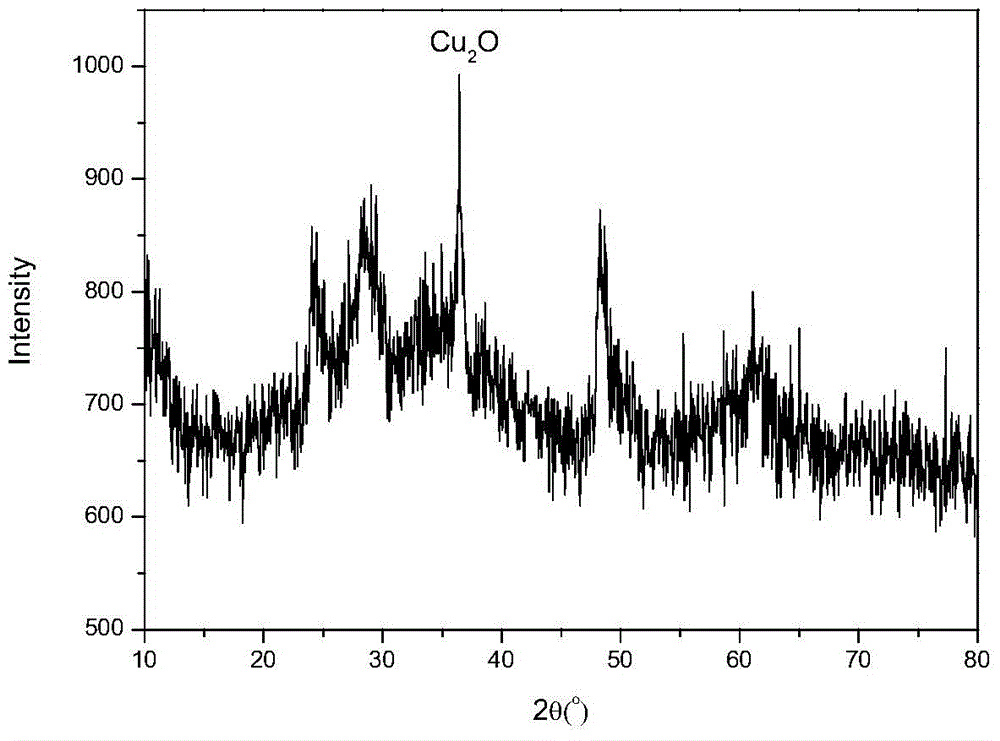
[0027] Example 2Cu 2 O loaded TiO 2 Preparation of nanotubes
[0028] (1) 1.2g TiO 2 (P25) with 120mL 10mol L -1 mixed NaOH solution, stirred for 30min, transferred to a 150mL hydrothermal kettle with Teflon lining, and reacted hydrothermally at 150°C for 24 hours;
[0029] (2) Cool to room temperature, pour off the supernatant, centrifuge to obtain a solid, wash three times with 100mL absolute ethanol, and centrifuge to obtain a solid;
[0030] (3) Control the mol ratio of glucose and copper acetate to be 1 / 16, add in the ethanol solution of 0.02g morphology modifier polyethylene glycol, stir and pour into the hydrothermal kettle with polytetrafluoroethylene liner at 130 ℃ for 24 hours;
[0031] (4) Cool naturally, then wash off the organic matter and sodium ions on the surface with absolute ethanol and water. At this time, the pH value is about 7. Finally, the solid is vacuum-dried at 80°C for 5 hours and ground to obtain Cu 2 O loaded TiO 2 nanotube.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com