Pharmaceutical composition for treating cystitis
A technique for cystitis and bladder perfusion, applied in the field of cystitis treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
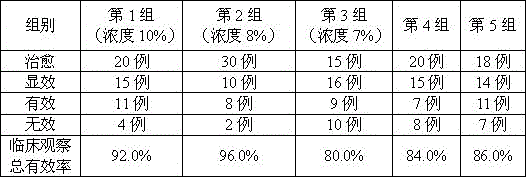
Embodiment 1
[0021] The preparation of embodiment 1 collagen perfusate
[0022] Collagen
[0023] Preparation: Make liquid under nitrogen-filled conditions, measure 90% volume of water for injection in the liquid mixing tank, weigh the prescription amount of sodium chloride, disodium hydrogen phosphate, and sodium dihydrogen phosphate, add it to the water, stir for 15 minutes to complete the dissolution Finally, add collagen, stir for 15 minutes to complete the dissolution, add water to the full volume, add 0.05% (w / v total volume) of activated carbon, absorb at 40-60°C for 15 minutes, decarbonize, and pass through a 0.22 μm filter membrane. Determination of intermediates, 95.0% of the labeled amount, so that the pH value is within the range of 7.2±0.1, filled with nitrogen, filled (50ml / bottle), melt-sealed, sterilized, sterilized at 115°C for 30 minutes, inspected by light, and bottled .
Embodiment 2
[0024] The preparation of embodiment 2 collagen perfusate
[0025] Collagen
[0026] Preparation: Make liquid under nitrogen-filled conditions, measure 90% volume of water for injection in the liquid mixing tank, weigh the prescription amount of sodium chloride, disodium hydrogen phosphate, and sodium dihydrogen phosphate, add it to the water, stir for 15 minutes to complete the dissolution Finally, add collagen, stir for 15 minutes to complete the dissolution, add water to the full volume, add 0.05% (w / v total volume) of activated carbon, absorb at 40-60°C for 15 minutes, decarbonize, and pass through a 0.22 μm filter membrane. Determination of intermediates is 105.0% of the labeled amount, so that the pH value is within the range of 7.2±0.1, filled with nitrogen, filled (50ml / bottle), melt-sealed, sterilized, sterilized at 115°C for 30 minutes, inspected by light, and bottled .
Embodiment 3
[0027] The preparation of embodiment 3 collagen perfusate
[0028] Collagen
[0029] Preparation: Make liquid under nitrogen-filled conditions, measure 90% volume of water for injection in the liquid mixing tank, weigh the prescription amount of sodium chloride, disodium hydrogen phosphate, and sodium dihydrogen phosphate, add it to the water, stir for 15 minutes to complete the dissolution Finally, add collagen, stir for 15 minutes to complete the dissolution, add water to the full volume, add 0.05% (w / v total volume) of activated carbon, absorb at 40-60°C for 15 minutes, decarbonize, and pass through a 0.22 μm filter membrane. Determination of intermediates, 104.1% of the labeled amount, so that the pH value is within the range of 7.2±0.1, nitrogen filling, filling (50ml / bottle), melting sealing, sterilization, sterilizing at 115°C for 30 minutes, light inspection, and bottling .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com