Production process for preparing astragalus polysaccharide liposome nano-preparation
A technology for the production of astragalus polysaccharide and its production process, which is applied in the field of production process for the preparation of astragalus polysaccharide liposome nano-preparation, and can solve the problems of difficult to meet market demand, low drug efficacy, low production efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
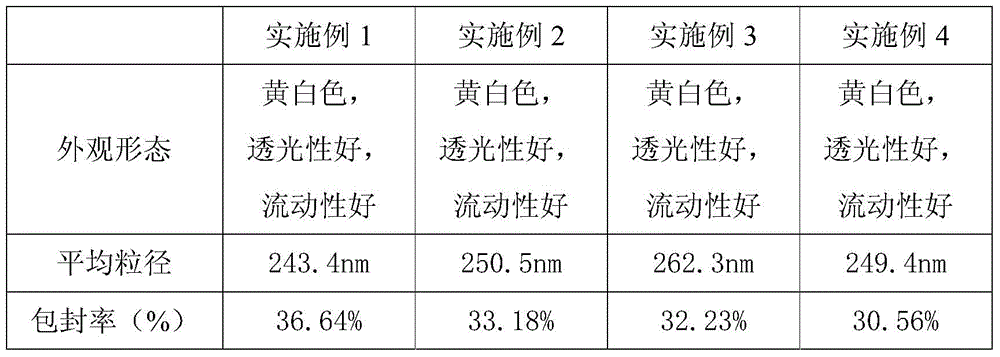
Embodiment 1
[0014] Accurately weigh analytically pure soybean lecithin (300mg) and cholesterol (75mg) according to the mass ratio of 4:1, and dissolve them in absolute ethanol (4ml) to make a lipid solution with a mass concentration of 7.5%;
[0015] Dissolve astragalus polysaccharide (100mg) with pH 7.0 phosphate buffer (25ml) and stir (55°C constant temperature, 1800r / min) to make astragalus polysaccharide solution with a mass concentration of 0.4%;
[0016] While stirring the astragalus polysaccharide solution obtained in step , slowly inject the lipid solution obtained in step at a volume ratio of 3:1, using a No. 5 syringe needle and keeping the needle below the liquid level, continue Stir for 1 hour, remove ethanol by volatilization to obtain astragalus polysaccharide liposome suspension;
[0017] Pass the astragalus polysaccharide liposome suspension obtained in step sequentially through 0.45 μm and 0.22 μm filter membranes to obtain a transparent milky liquid, which is store...
Embodiment 2
[0019] Accurately weigh analytically pure soybean lecithin (300mg) and cholesterol (150mg) according to the mass ratio of 2:1, and dissolve them in absolute ethanol (4ml) to make a lipid solution with a mass concentration of 15%;
[0020] Dissolve astragalus polysaccharide (100mg) with pH 7.5 phosphate buffer (25ml) and stir (65°C constant temperature, 1500r / min) to make astragalus polysaccharide solution with a mass concentration of 1.0%;
[0021] While stirring the astragalus polysaccharide solution obtained in step , slowly inject the lipid solution obtained in step at a volume ratio of 4:1, using a No. 5 syringe needle and keeping the needle below the liquid level, continue Stir for 1 hour, remove ethanol by volatilization to obtain astragalus polysaccharide liposome suspension;
[0022] Pass the astragalus polysaccharide liposome suspension obtained in step sequentially through 0.45 μm and 0.22 μm filter membranes to obtain a transparent milky liquid, which is store...
Embodiment 3
[0024] Accurately weigh analytically pure soybean lecithin (300mg) and cholesterol (50mg) according to the mass ratio of 6:1, and dissolve them in absolute ethanol (4ml) to make a lipid solution with a mass concentration of 10%;
[0025] Dissolve astragalus polysaccharide (100mg) with pH 7.5 phosphate buffer (25ml) and stir (45°C constant temperature, 1600r / min) to make astragalus polysaccharide solution with a mass concentration of 0.3%;
[0026] While stirring the astragalus polysaccharide solution obtained in step , slowly inject the lipid solution obtained in step at a volume ratio of 5:1, using a No. 5 syringe needle and keeping the needle below the liquid level, continue Stir for 1 hour, remove ethanol by volatilization to obtain astragalus polysaccharide liposome suspension;
[0027] Pass the astragalus polysaccharide liposome suspension obtained in step sequentially through 0.45 μm and 0.22 μm filter membranes to obtain a transparent milky liquid, which is stored...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com