Novel synthesis method for L-menthyl-N,N-dimethyl succinamide
A technology of dimethylsuccinamide and synthesis method is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc., and can solve the problems of high production cost, low reaction yield, complicated post-processing and the like, and achieves The three wastes are small, the purity and yield are improved, and the post-processing is easy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
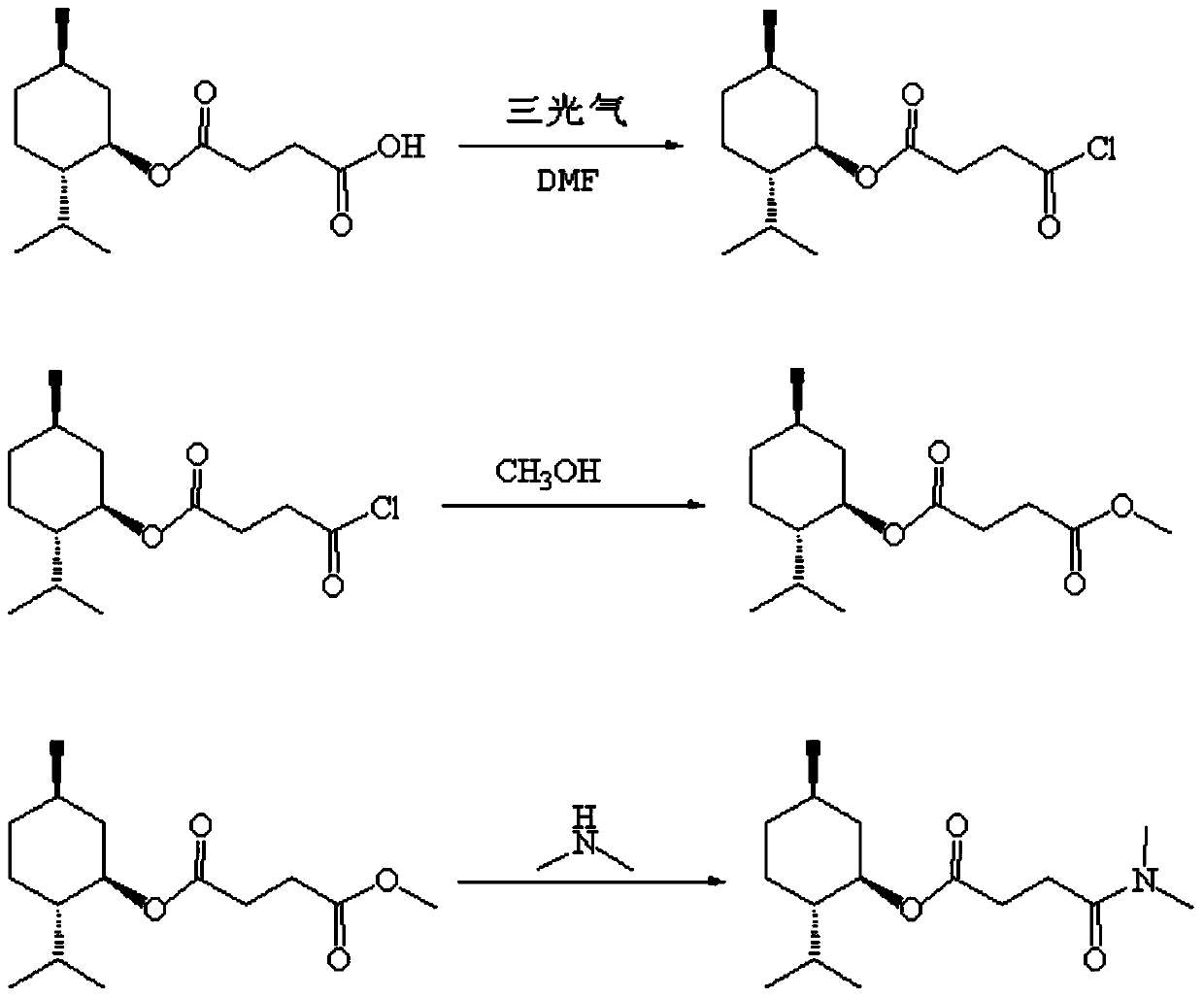
[0016] (1) Preparation of Menthyl Succinic Acid Monochloride
[0017] Add 1mol L-monomenthyl succinate, 0.005mol DMF catalyst, 3.5mol 1,2-dichloroethane into a 2000ml four-necked round bottom flask, and add dropwise triphosgene solution (0.3mol triphosgene dissolved in 0.24mol1,2-dichloroethane), keep the internal temperature at 83°C during the dropwise addition, stir and react for 30h; stop the reaction to obtain menthyl succinic acid chloride.
[0018] (2) Preparation of menthyl methyl succinate
[0019] Gained menthyl succinic acid chloride, the control temperature is 50 ℃. Add 1 mol of methanol into a constant pressure titration funnel, and slowly drop it into the prepared menthyl succinic monochloride reaction solution. During the dropping process, control the temperature at 50°C, stir for 20 hours, add water to destroy the system, and let it stand for water separation. The water phase was extracted by adding 1,2-dichloroethane, and the oil phases were combined. Adjust...
Embodiment 2
[0023] This example relates to a new method for the synthesis of L-menthyl-N,N-dimethylsuccinamide
[0024] (1) Preparation of Menthyl Succinic Acid Monochloride
[0025] Add 1mol L-monomenthyl succinate, 0.1mol DMF catalyst, 1mol1,2-dichloroethane in a 2000ml four-neck round bottom flask, and drop triphosgene solution (3mol triphosgene dissolved in 9mol1, 2-dichloroethane), maintain the internal temperature at 15°C during the dropwise addition, and stir the reaction for 20 minutes; stop the reaction to obtain menthyl succinic acid chloride.
[0026] (2) Preparation of menthyl methyl succinate
[0027] The resulting menthyl succinic acid chloride was cooled to -30°C. Add 50 mol of methanol into a constant pressure titration funnel, and slowly drop it into the prepared menthyl succinic monochloride reaction solution. During the dropping process, control the temperature at -30°C, stir for 30 minutes, add water to destroy the system, and let stand to separate the water. The wa...
Embodiment 3
[0031] This example relates to a new method for the synthesis of L-menthyl-N,N-dimethylsuccinamide
[0032] (1) Preparation of Menthyl Succinic Acid Monochloride
[0033] Add 1mol L-monomenthyl succinate, 0.02mol DMF catalyst, 2.5mol1,2-dichloroethane into a 2000ml four-neck round bottom flask, and add triphosgene solution (2mol triphosgene dissolved in 5mol1 , 2-dichloroethane), maintain the internal temperature at 50°C during the dropwise addition, and stir the reaction for 16h; stop the reaction to obtain menthyl succinic acid chloride.
[0034] (2) Preparation of menthyl methyl succinate
[0035] The resulting menthyl succinic acid chloride was cooled to 0°C. Add 24 mol of methanol into a constant pressure titration funnel, and slowly drop it into the prepared menthyl succinic monochloride reaction solution. During the dropwise addition, control the temperature at 0°C, stir for 13 hours, add water to destroy the system, and let stand to separate the water. The water pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com