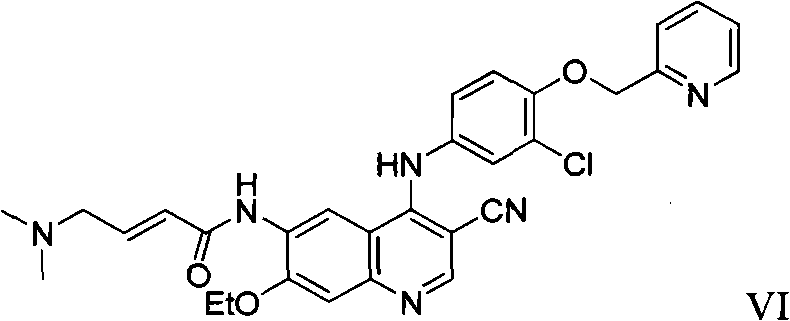
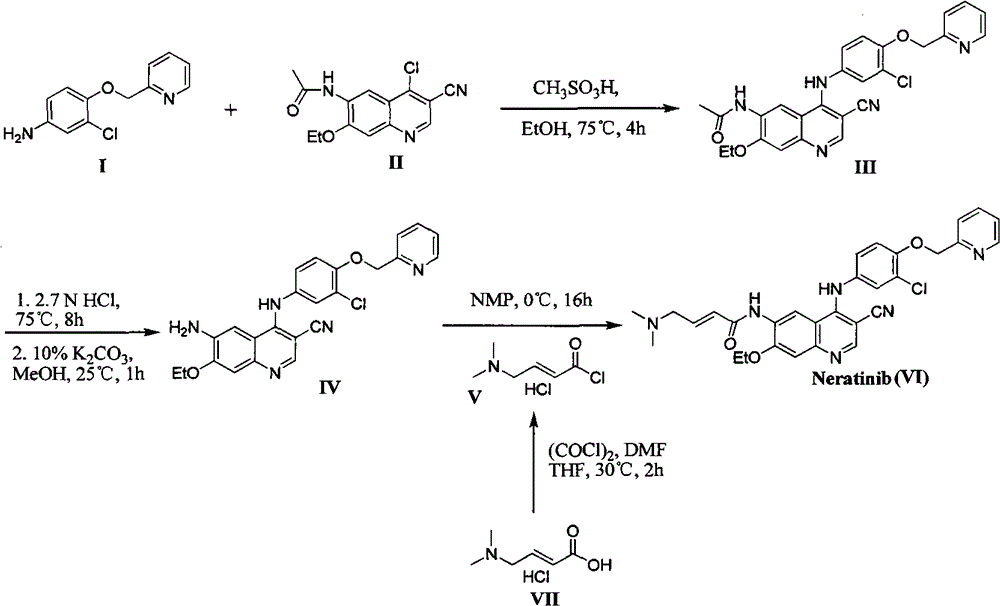
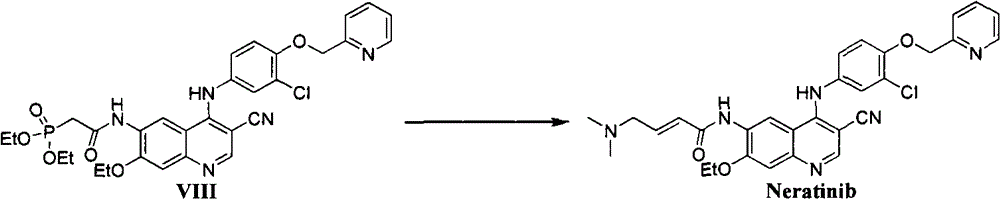
The preparation method of neratinib
A compound and molar ratio technology, applied in the field of preparation of the anticancer drug Neratinib, can solve the problems of high toxicity, environmental pollution and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthesis of embodiment 1Neratinib
[0023] N 2 Under protection, 11.7mL (0.07mol) of 6mol / L hydrochloric acid was cooled to 0°C, 9.1mL (0.05mol) of dimethylaminoacetaldehyde diethyl acetal was added dropwise, hydrolyzed at 40°C for 12 hours, cooled to 0°C, and set aside Solution A).
[0024] Add N-[4-[[3-chloro-4-(2-pyridylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinone in sequence to 150mL absolute ethanol Phenyl]-2-diethyl phosphate-acetamide 12.5g (0.02mol), lithium chloride 0.85g (0.02mol), lower the temperature to 0°C, add sodium ethoxide 4.1g (0.06mol), stir for 0.5h, drop Add stock solution A, and continue to react for 40 minutes after dropping. Add 500 mL of ethyl acetate, filter, and add 500 mL of water to the filtrate. Separate the organic layer, extract the aqueous layer with 2×200 mL ethyl acetate, combine the organic layers, wash with water, wash with saturated brine, and dry over anhydrous sodium sulfate. Concentrate to dryness under reduced pre...
Embodiment 2
[0027] The synthesis of embodiment 2Neratinib
[0028] Add N-[4-[[3-chloro-4-(2-pyridylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolinyl successively to 150mL methanol ]-2-diethyl phosphate-acetamide 12.5g (0.02mol), lithium chloride 0.85g (0.02mol), cooled to -10°C, added sodium methoxide 5.4g (0.1mol), stirred for 0.5h, added dropwise The standby solution A described in Example 1 continued to react for 40 minutes after dropping. Add 500 mL of ethyl acetate, filter, and add 500 mL of water to the filtrate. Separate the organic layer, extract the aqueous layer with 2×200 mL ethyl acetate, combine the organic layers, wash with water, wash with saturated brine, and dry over anhydrous sodium sulfate. Concentrate to dryness under reduced pressure and recrystallize from acetonitrile / THF to obtain 9.5 g of light yellow solid with a yield of 85.1%.
Embodiment 3
[0029] The synthesis of embodiment 3Neratinib
[0030] Add N-[4-[[3-chloro-4-(2-pyridylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolone successively to 50mL isopropanol Phenyl]-2-diethyl phosphate-acetamide 1.25g (0.002mol), lithium chloride 0.17g (0.004mol), lower the temperature to 0°C, add sodium isopropoxide 0.82g (0.01mol), stir for 1h, Add 0.005 mol of dimethylaminoacetaldehyde diethyl acetal dropwise to prepare solution A, and continue to react for 3 hours after dropping. Add 200 mL of ethyl acetate, filter, and add 200 mL of water to the filtrate. Separate the organic layer, extract the aqueous layer with 2×50 mL ethyl acetate, combine the organic layers, wash with water, wash with saturated brine, and dry over anhydrous sodium sulfate. Concentrate to dryness under reduced pressure and recrystallize from acetonitrile / THF to obtain 0.92 g of light yellow solid with a yield of 82.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com