Antibacterial compound containing quinoline or pyrazole heterocycle rhodanine structure
A compound, low-level technology, applied in antibacterial drugs, organic chemistry, etc., can solve problems such as increasing the difficulty of treating infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
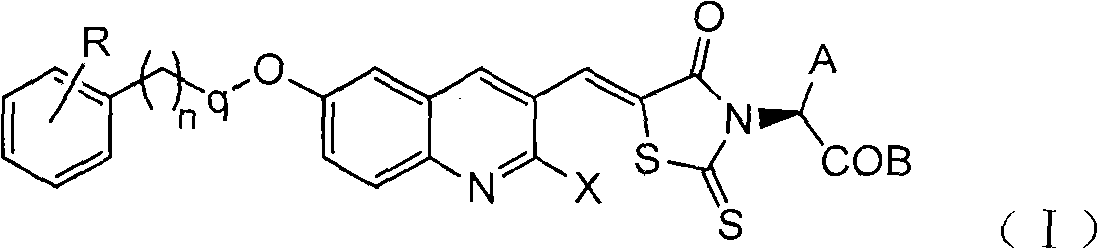
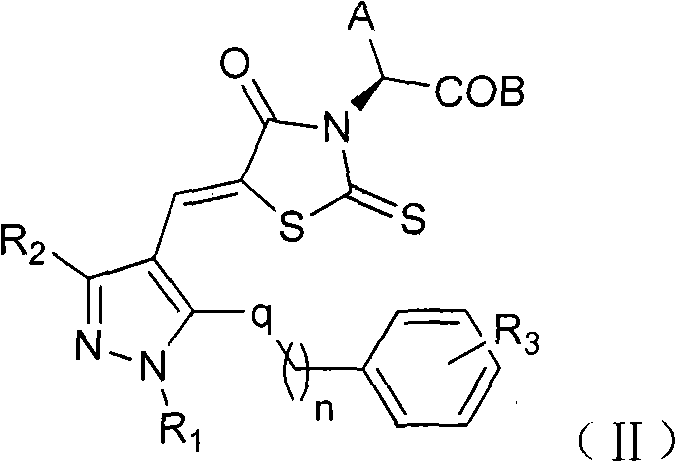
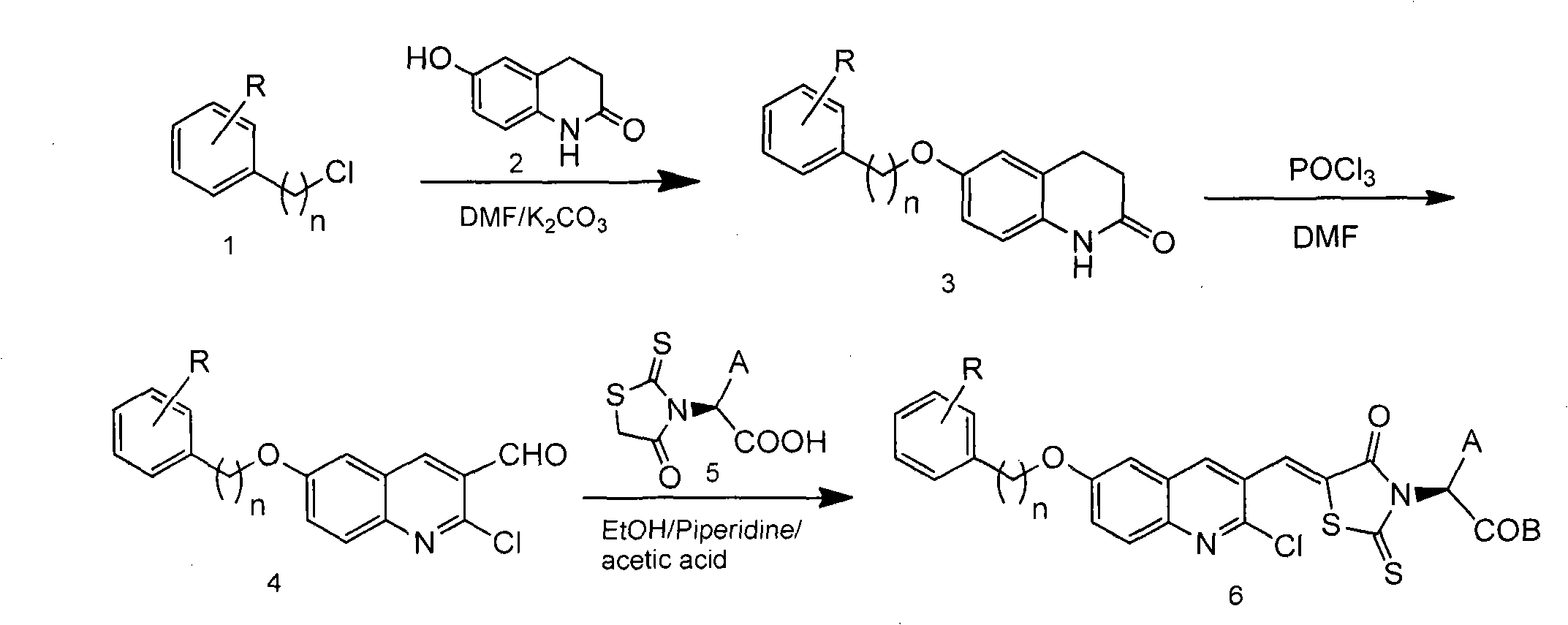
[0038] With (Z)-2-(5-((2-chloro-6-((4-methylbenzyl)oxy)quinolin-3-yl)methylene)-4-oxo-2-thiazolidinone Taking -3-yl)-3-phenylpropionic acid as an example to introduce the general synthesis method of compound (Ⅰ)
[0039] (1) Add 6-hydroxyquinolinone and an equivalent amount of p-methylbenzyl chloride into a 100mL round-bottomed flask, use N,N-dimethylformamide (DMF) as a solvent, add 2 times the amount of potassium carbonate, 70-80 °C for 3 hours. The reaction solution was poured into an appropriate amount of ice water, filtered, washed with water, and dried to obtain compound 3.
[0040] (2) Add 10mL DMF to a 100mL round-bottomed flask, cool it down to below 5°C with an ice-water bath, add 3g of distilled phosphorus oxychloride dropwise, and after the dropwise addition, fully stir at this temperature for 15 minutes to dissolve compound 3 Dissolve in 6 mL of DMF, add dropwise to the above mixture, stir at below 5°C for half an hour, then raise the temperature to 70°C, and st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com