Preparation method of deferasirox derivative
A technology of derivatives and products, applied in the field of medicine, can solve the problems of complex synthetic routes of deferasirox derivatives, difficult purification and purification, low total synthesis yield, etc., achieves convenient purification, low production cost, and overcomes separation and purification. cumbersome effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
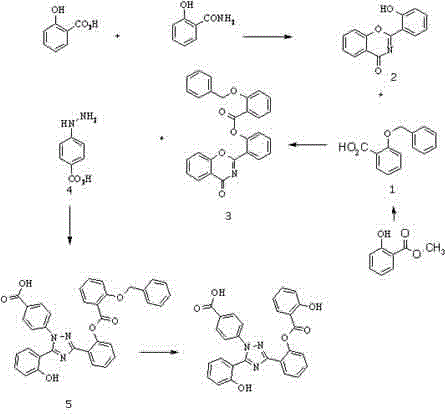
[0024] Example 1 Preparation of Derasirox Derivatives
[0025] like figure 1 Shown:
[0026] (1) Dissolve methyl salicylate (0.046mol) in dry dimethylformamide solvent (56ml), add potassium hydroxide (0.185mol) at low temperature, then add benzyl chloride (0.046mol) dropwise, After the reaction was stirred at room temperature for 4 hours, 100ml of water was added. After the reaction mixture was stirred for 1 hour, the pH was adjusted to 1 with acid in an ice bath, extracted with ethyl acetate (200mlx3), and the organic phase was dried with anhydrous sodium sulfate and evaporated. Drying yielded 8.5 g of white solid intermediate 1 (benzylsalicylic acid); used directly in the next reaction.
[0027] (2) Suspend 8.5 g of intermediate product 1 prepared in step (1) in 21 ml of thionyl chloride, reflux for 4 hours, and evaporate to dryness to obtain 7.5 g of oil. Compound 2, (2-(2-hydroxyphenyl)-4H-1,3-benzoxazin-4-one) (0.023mol) was suspended in 57ml of dry dichloromethane and...
Embodiment 2
[0033] The deferasirox derivative prepared in Example 1 of the present invention was used in 100 patients with iron overload caused by blood transfusion. Experiments showed that the effective rate was 100%, and no adverse reactions were observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com