Phenanthrene quinoxaline fluorescent compound and preparation method and application thereof, and electroluminescent device
A technology for electroluminescent devices and fluorescent compounds, which is applied in the fields of electro-solid devices, chemical instruments and methods, electrical components, etc., can solve the problems of red shift of the emission spectrum of fluorescent devices, low hole and electron transfer rate, etc. Efficiency, redshift reduction, and stability enhancement effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
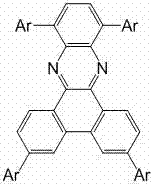
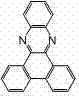
[0044] When Ar = , the phenanthroxaline fluorescent compound is 3,6,10,13-tetraphenylphenanthroxaline (abbreviated as TPhPhZN), and its molecular structure is as follows: Figure 6 shown.
[0045] Its preparation method comprises the following steps:
[0046] a. Dissolve 3,6-dibromo-o-phenylenediamine (10.0 mmol), 3,6-dibromo-9,10-phenanthrenequinone (10.0 mmol) and sodium hydroxide (25.0 mmol) in absolute ethanol (50.0 ml), heated to 100°C and refluxed for 4 hours, a yellow solid precipitated out slowly, after the reaction was complete, cooled to room temperature, and filtered directly, the obtained yellow solid 3,6,10,13-tetrabromophenanthroxaline, Drying, directly used in the next reaction, yield: 95%. MS (APCI): theoretical value C 20 h 8 Br 4 N 2 : 591.7, experimental value, 593.2 (M+1) + .
[0047] b. Dissolve the solid 3,6,10,13-tetrabromophenanthroxaline (5.0 mmol) and phenylboronic acid (25.0 mmol) obtained above in toluene solution, and add potassium carbon...
Embodiment 2
[0049] When Ar = , the phenanthroxaline fluorescent compound is 3,6,10,13-tetrakis(1-naphthyl)phenanthroxaline (abbreviated as 1-TNaPhZN), and its molecular structure is as follows: Figure 7 shown.
[0050] Its preparation method comprises the following steps:
[0051] a. Dissolve 3,6-dibromo-o-phenylenediamine (10.0 mmol), 3,6-dibromo-9,10-phenanthrenequinone (10.0 mmol) and sodium hydroxide (25.0 mmol) in absolute ethanol (50.0 ml), heated to 100°C and refluxed for 4 hours, a yellow solid precipitated out slowly, after the reaction was complete, cooled to room temperature, and filtered directly, the obtained yellow solid 3,6,10,13-tetrabromophenanthroxaline, Drying, directly used in the next reaction, yield: 95%. MS (APCI): theoretical value C 20 h 8 Br 4 N 2 : 591.7, experimental value, 593.2 (M+1) + .
[0052] b. The solid 3,6,10,13-tetrabromophenanthroxaline (5.0 mmol) obtained above, 1- Naphthaleneboronic acid (25.0 mmol) was dissolved in toluene solution, po...
Embodiment 3
[0054] When Ar = , the phenanthroxaline fluorescent compound is 3,6,10,13-tetrakis(2-naphthyl)phenanthroxaline (abbreviated as 2-TNaPhZN), and its molecular structure is as follows: Figure 8 shown.
[0055] Its preparation method comprises the following steps:
[0056] a. Dissolve 3,6-dibromo-o-phenylenediamine (10.0 mmol), 3,6-dibromo-9,10-phenanthrenequinone (10.0 mmol) and sodium hydroxide (25.0 mmol) in absolute ethanol (50.0 ml), heated to 100°C and refluxed for 4 hours, a yellow solid precipitated out slowly, after the reaction was complete, cooled to room temperature, and filtered directly, the obtained yellow solid 3,6,10,13-tetrabromophenanthroxaline, Drying, directly used in the next reaction, yield: 95%. MS (APCI): theoretical value C 20 h 8 Br 4 N 2 : 591.7, experimental value, 593.2 (M+1) + .
[0057] b. The solid 3,6,10,13-tetrabromophenanthroxaline (5.0 mmol) obtained above, 2- Naphthaleneboronic acid (25.0 mmol) was dissolved in toluene solution, po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com