Synthesis method of nucleoside diphosphate 6-deoxy-L-pyranose
A nucleoside diphosphate and synthesis method technology, applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve the problems of lack of product terminal stereo configuration, low reaction yield, difficult to remove, etc., to achieve The effect of high practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
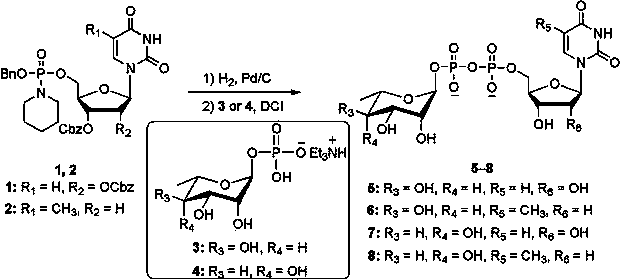
[0012] Uridine 5′-diphosphate-α-L-rhamnose disodium salt 5 The synthesis of N -( O -Benzyl- O -(2′,3′-dibenzyloxycarbonyl)uridine-5′-)phosphorylpiperidine 1 (100 mg, 0.13 mmol), 10% palladium carbon (5 mg, 5% by weight) and triethylamine (18 μL, 0.13 mmol) were dissolved in dry N , N - Dimethylformamide (1 mL), hydrogenated at 20 °C for 3 hours under normal pressure. After hydrogenation, palladium carbon was removed using a filter membrane with a pore size of 0.45 μm, and the solution was added with α-L-rhamnose-1-monophosphate (triethylamine) salt (67 mg, 0.195 mmol), 4,5-dicyanoimidazole ( 61 mg, 0.52 mmol) was stirred at 30°C for 6 hours. After the reaction, the solvent was concentrated. Sodium acetate aqueous solution (10 M, 0.5 mL) was added to the residue to dissolve it, and ethanol (50 mL) was added to precipitate the product, and centrifuged to obtain crude uridine 5′-diphosphate-α-L-rhamnose sodium salt. Using reversed-phase HPLC (XTerra Prep MS C18 OBD TM 1...
Embodiment 2
[0014] Thymidine 5′-diphosphate-α-L-rhamnose disodium salt 6 The synthesis of N -( O -Benzyl- O -(3′-Benzyloxycarbonyl)thymidine-5′-)phosphorylpiperidine 2 (80 mg, 0.13 mmol), 5% palladium carbon (8 mg, 10% by weight) and tri-n-propylamine (25 μL, 0.13 mmol) were dissolved in dry N , N - Dimethylformamide (1 mL), hydrogenated at 30 °C for 2 hours under normal pressure. After hydrogenation, palladium carbon was removed using a filter membrane with a pore size of 0.45 μm, and the solution was added with α-L-rhamnose-1-monophosphate (triethylamine) salt (67 mg, 0.195 mmol), 4,5-dicyanoimidazole ( 61 mg, 0.52 mmol) was stirred at 40°C for 4 hours. After the reaction, the solvent was concentrated. Add sodium acetate aqueous solution (10 M, 0.5 mL) to the residue to dissolve, add ethanol (50 mL) to precipitate the product, and centrifuge to obtain the crude thymidine 5′-diphosphate-α-L-rhamnose sodium salt. Using reversed-phase HPLC (XTerra Prep MS C18 OBD TM 10 μm, 19 × 2...
Embodiment 3
[0016] Uridine 5′-diphosphate-6-deoxy-α-L-talose disodium salt 7 The synthesis of N -( O -Benzyl- O -(2′,3′-dibenzyloxycarbonyl)uridine-5′-)phosphorylpiperidine 1 (100 mg, 0.13 mmol), 10% palladium on carbon (10 mg, 10% by weight) and diisopropylethylamine (21 μL, 0.13 mmol) were dissolved in dry N , N -Dimethylformamide (1 mL), hydrogenated at 40 °C for 1 hour under normal pressure. After hydrogenation, palladium carbon was removed using a filter membrane with a pore size of 0.45 μm, and the solution was added with 6-deoxy-α-L-talose-1-monophosphate (triethylamine) salt (89 mg, 0.26 mmol), 4,5-di Cyanoimidazole (77 mg, 0.65 mmol) was stirred at 30°C for 6 hours. After the reaction, the solvent was concentrated. Sodium acetate aqueous solution (10 M, 0.5 mL) was added to the residue to dissolve it, and then ethanol (50 mL) was added to precipitate the product, and centrifuged to obtain crude uridine 5′-diphosphate-6-deoxy-α-L-talose sodium salt. Using reversed-phase HP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com