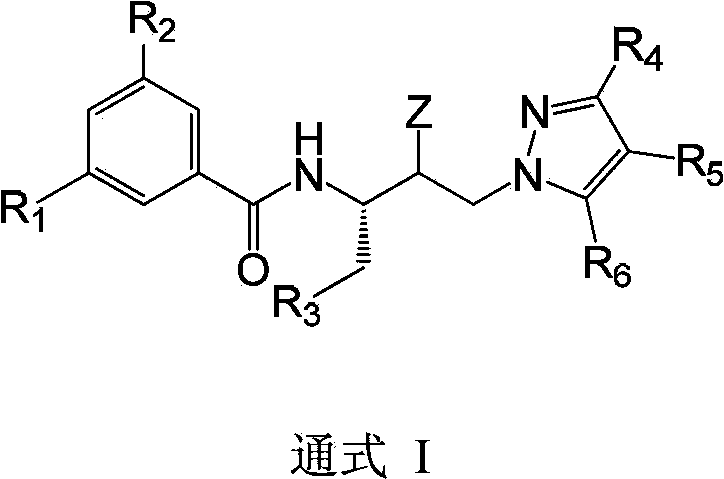
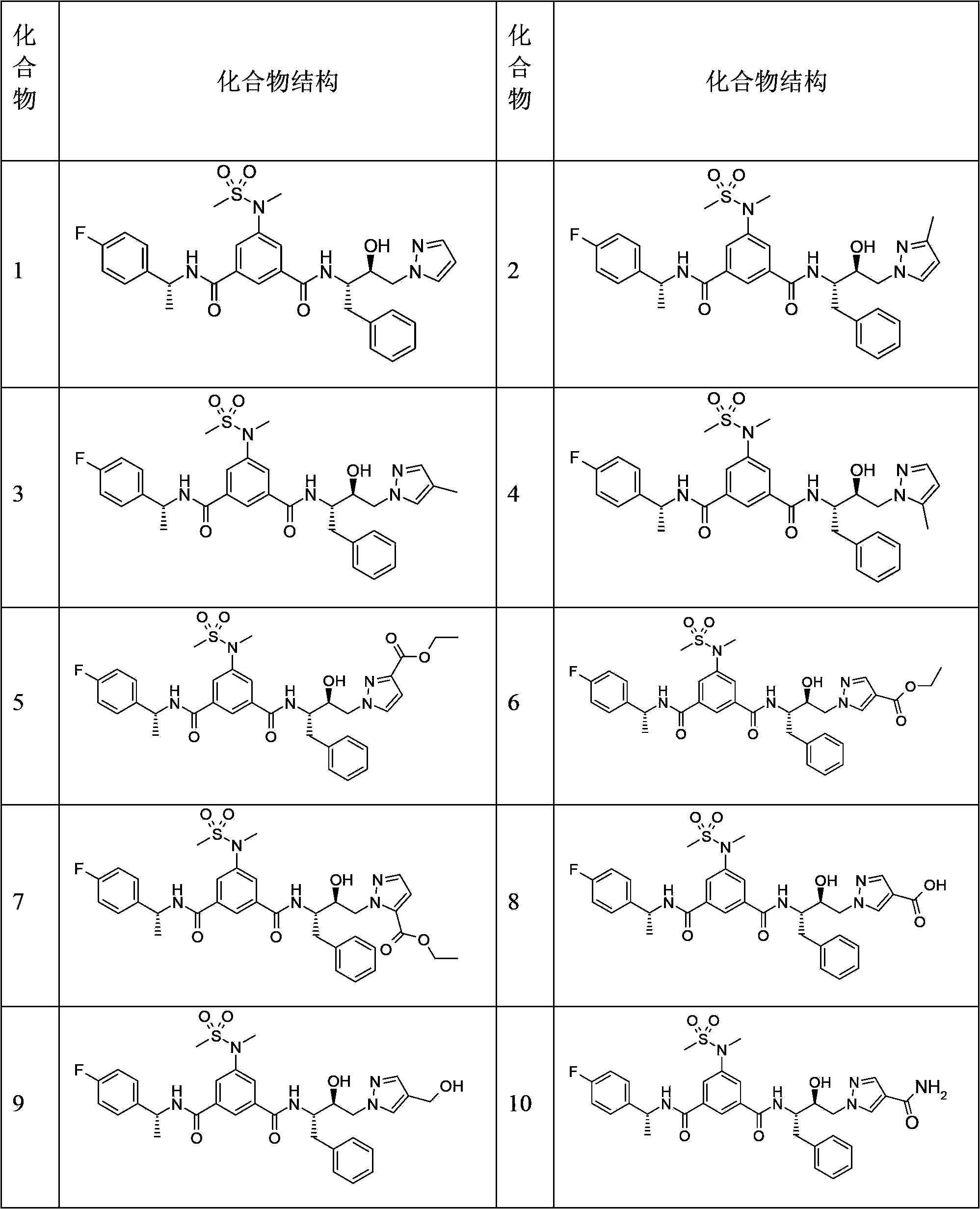
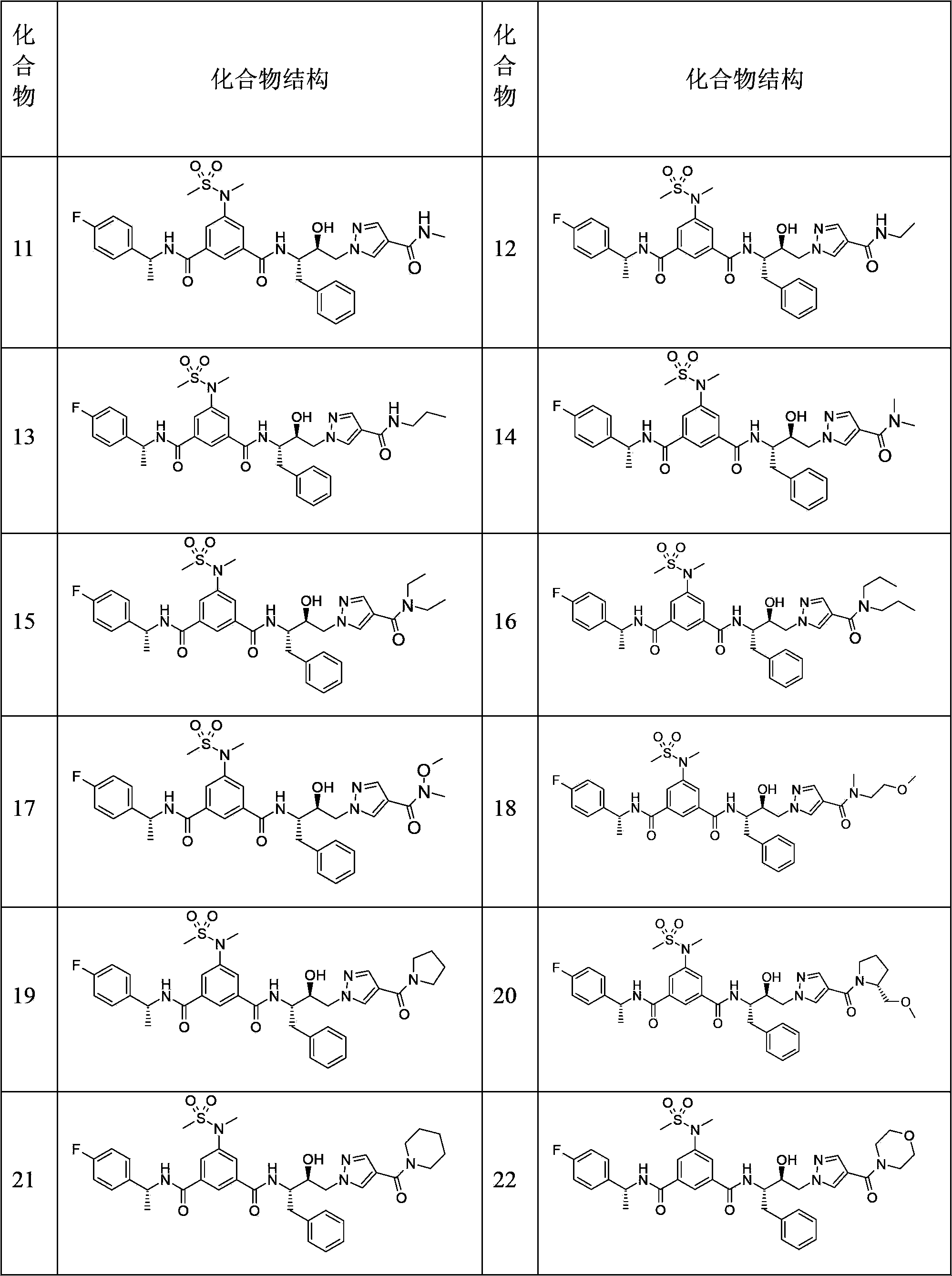
Hydroxyethyl pyrazole compound or aminoethyl pyrazole compound, preparation method and use thereof
A technology of aminoethylpyrazoles and hydroxyethylpyrazoles, which is applied in the field of drug synthesis and can solve the problems of high synthesis difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] (Preparation of compound 1)
[0129] Synthetic route 1, reaction steps:
[0130] The epoxy compound (2S,3S)-1,2-epoxy-3-(N-tert-butoxycarbonylamino)-4-phenylpropane (50mg, 0.19mmol) was dissolved in 2mL DMF, and pyrazole was added to the system (13mg, 0.19mmol), catalytic amount of sodium carbonate. React in microwave at 110°C for 1 hour. At the end of the reaction, add 25 mL of water to the system, extract with ethyl acetate (25 mL×2), combine the organic phases, wash with saturated brine (25 mL), dry over anhydrous sodium sulfate, filter, and concentrate to obtain a white solid (2S,3S)-1 - tert-butyl benzyl-2-hydroxy-3-pyrazolylcarbamate 57 mg,
[0131] The above pyrazole compound (57 mg, 0.17 mmol) was dissolved in 4 mL of dichloromethane, 1 mL of trifluoroacetic acid was added to the system, and reacted at room temperature for 2 h. After concentration, the obtained amine was directly used in the next reaction without treatment. Dissolve the amine obtained in ...
Embodiment 2
[0135] (Preparation of Compound 2)
[0136] The synthetic route refers to Example 1. Using 3-methylpyrazole instead of pyrazole, TLC chromatography gave white amorphous solid compound 2 (19 mg, yield: 81.2%).
[0137] 1 H NMR (300MHz, CD 3 OD):δ8.13(s,1H),8.00(s,1H),7.90(s,1H),7.49(m,1H),7.44-7.39(m,2H),7.26-7.22(m,4H) ,7.17-7.13(d,1H),7.05-7.02(t,2H),6.01.(s,1H),5.24-5.21(q,J=7.2Hz,1H),4.39.-4.37(t,1H) ,4.19.-4.00(m,3H),3.34(s,3H),3.03-2.96(m,2H),2.94(s,3H),2.18(s,3H),1.58-1.55(d,J=7.2 Hz,3H)
[0138] LC-MS: m / z622.22[M+H] + ;
Embodiment 3
[0140] (Preparation of Compound 3)
[0141] Referring to Example 1 for the synthetic route, 4-methylpyrazole was used instead of pyrazole, and TLC chromatography gave white amorphous solid compound 3 (71 mg, yield: 72.4%).
[0142] 1 H NMR (300MHz, CD 3 OD): δ8.11(s,1H),7.99(s,1H),7.89(s,1H),7.44-7.38(m,3H),7.26-7.17(m,5H),7.16-7.14(d, 1H), 7.05-7.00(t, 2H), 5.24-5.20(q, J=6.6Hz, 1H), 4.39.-4.34(t, 1H), 4.20-4.16(m, 1H), 4.08-4.00(m ,2H),3.34(s,3H),3.05-3.00(m,2H),2.98(s,3H),2.02(s,3H),1.58-1.56(d,J=6.6Hz,3H)
[0143] LC-MS: m / z622.2[M+H] + ;
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com