Multiparticulate l-menthol formulations and related methods
A menthol and multiparticulate technology, applied in the field of high-purity L-multiparticulate preparations, can solve problems such as long residence time and unpredictable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0043] Example 1: Preparation of multiparticulate formulations
[0044] Experiment Details
[0045] Equipment used to manufacture the formulations described herein included: dish-on-dish balance, hand sieves (12, 14, 16, 18, weighing pan, 70 mesh screen), Rotap rotary sorting sieve, IKA mixer, KitchenAid Food Processor (Pre-Grind), Fitz Mill with 0.065'' Screen, Jet Mill, Key International High Shear Mixer, Glatt GPCC-3 Fluid Bed Dryer, Fluid Bed with 7'' Bottom Spray (wurster) Glatt GPCC-3 fluidized bed dryer, Karl Fischer moisture analyzer and spheronizer.
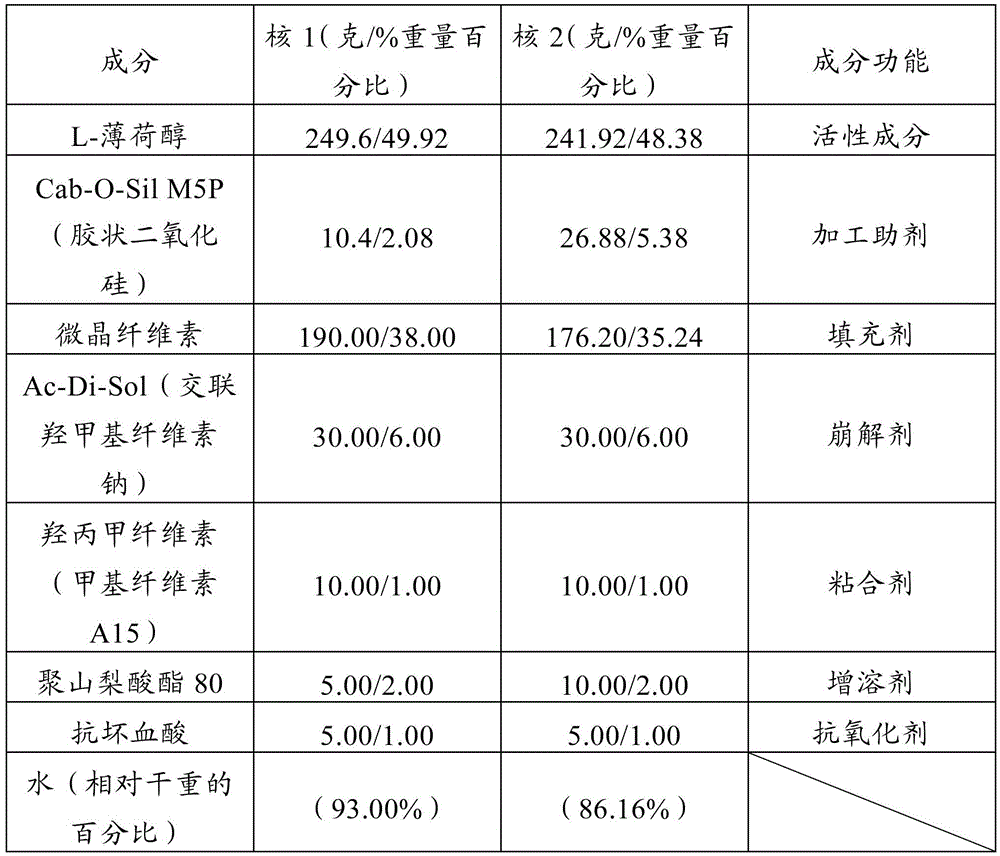
[0046] Preparation of Nucleus 1
[0047]As shown in Table 1, Core 1 was prepared as described above using the following settings. Wet granulation settings were: paddle speed 300 rpm, chopper speed 3450 rpm, wet granulation time 80 seconds to 90 seconds, maximum paddle power 5.5 amps to 6.2 amps. The extrusion settings were: paddle speed 25 rpm, feeder speed 30 rpm, screen diameter 1.2 mm. The extrudate was loaded to...
example 2
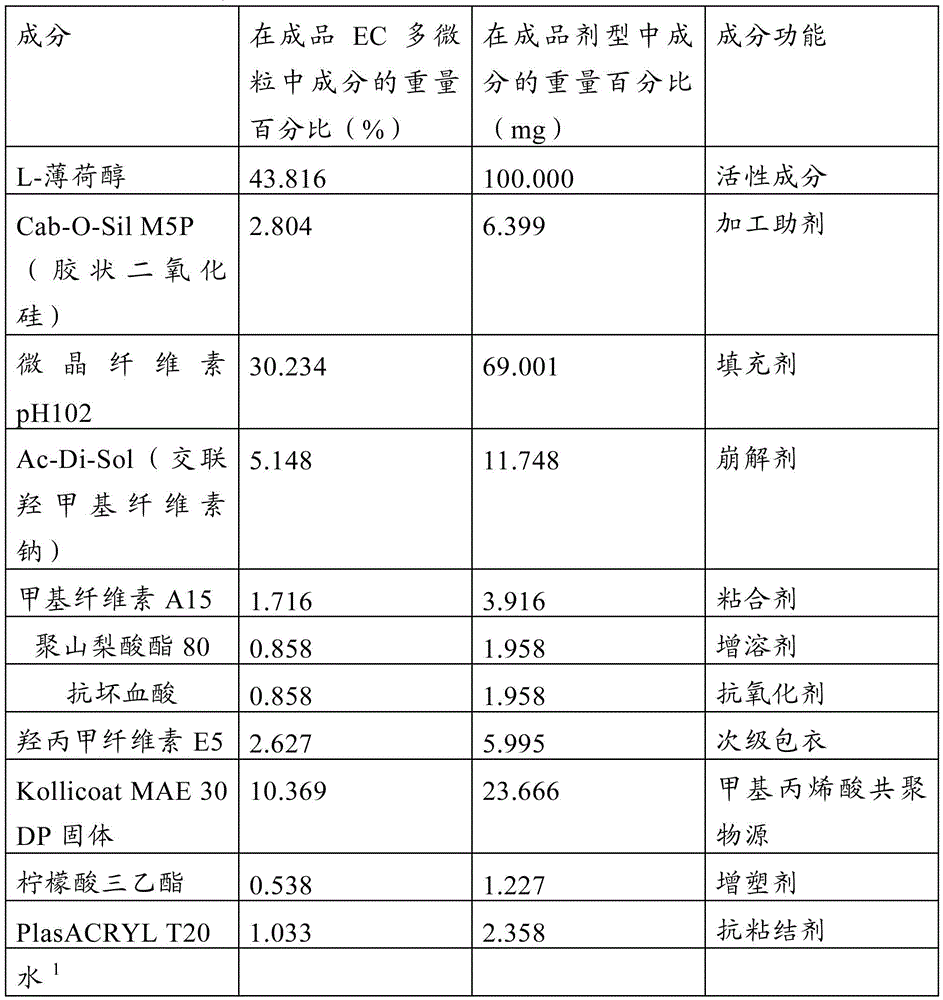
[0056] Example 2: Assays on prepared multiparticulate formulations
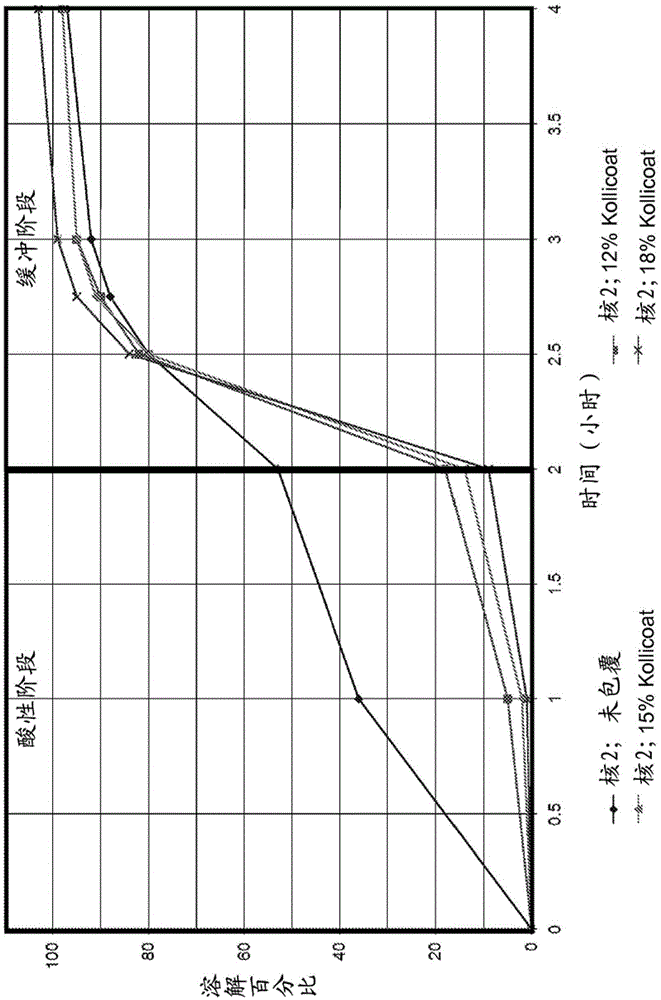
[0057] Prepared multiparticulate dosage forms were assayed to determine whether they met or approached the desired USP711 enteric specification. To meet the USP711 specification for enteric coating, less than 10% of the active ingredient should be released within 2 hours in 0.1N HCl solution (“acid phase”). Subsequently, not less than 85% of the active ingredient should be released within 45 minutes in a buffer solution with a pH value of 6.8 (“buffer phase”).
[0058] The data in Tables 5 and 6 indicate that both acrylic resin and Kollicoat coatings can be successfully used with crystalline L-menthol to form enteric-coated L-menthol multiparticulate formulations that meet or approach USP711 enteric specifications. In Tables 5 and 6, the batch identification indicates the core used, the coating on the core and the formulation number for the specific formulation. A secondary coating of 3% by weight hypromell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com