A kind of synthetic technique of temsirolimus
A technology of temsirolimus and synthesis process, applied in the direction of organic chemistry and the like, can solve the problems of low yield, lengthy reaction, increase production cost and the like, and achieve the effects of simple process operation, rapid reaction and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
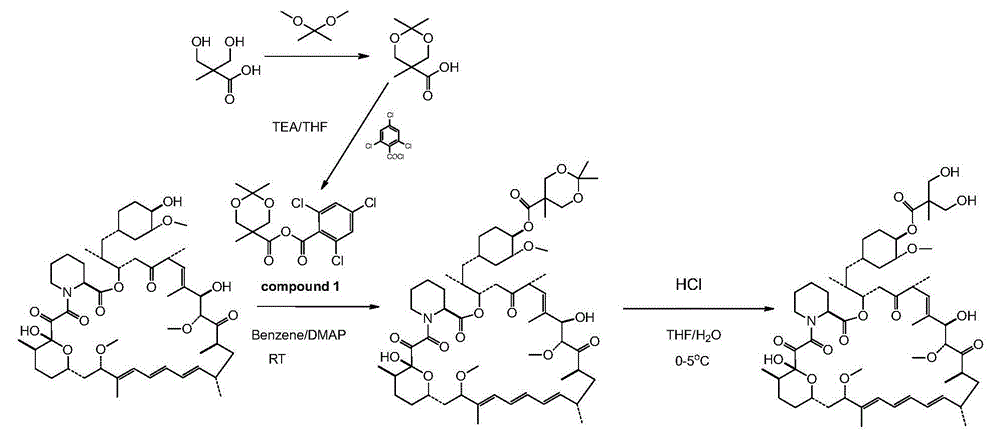
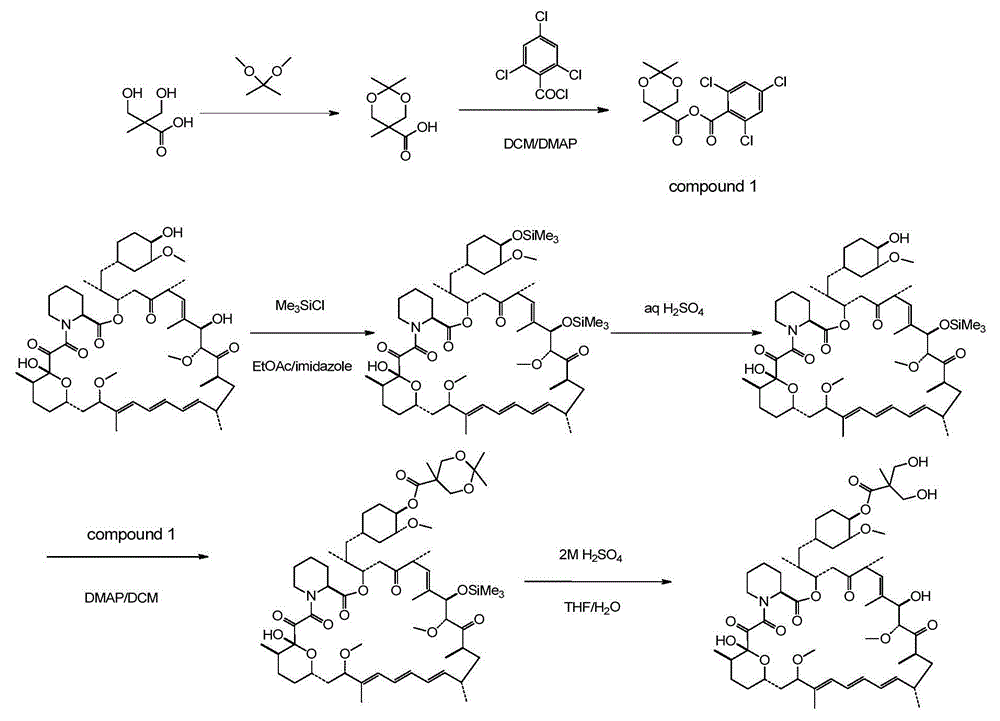
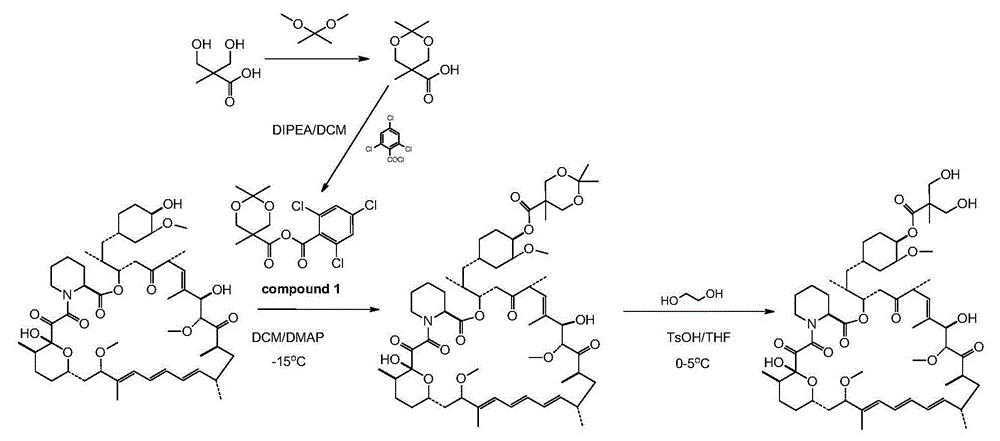
[0059] Step 1: Preparation of 2,2,5-trimethyl-5-carboxy-1,3-dioxane
[0060] In a 1L round bottom flask, add 100g of 2,2-dimethylhydroxy-propionic acid, 150g of 2,2-dimethoxypropane and 1.5g of p-toluenesulfonic acid in 400ml of anhydrous acetone and stir at room temperature After 5 hours, after adding 2ml of DIPEA, concentrated to dryness by rotary evaporation under reduced pressure to obtain a white solid, the resulting white solid was poured into 1L of dichloromethane and stirred mechanically for 30mins, filtered under reduced pressure, and the obtained filtrate was concentrated under reduced pressure to obtain 140g target product.
[0061] The second step: the preparation of acid anhydride
[0062] Put 48g of 2,2,5-trimethyl-5-carboxy-1,3-dioxane and 200ml of dichloromethane into a 1L three-necked bottle, and cool down to 0-5°C under nitrogen protection. Stir to dissolve, add 52g of DIPEA after dissolving, and dropwise add 63g of 2,4,6-trichlorobenzoyl chloride for 50-70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com