Levosulpiride compound and preparation method thereof
A levosulpiride compound technology, applied in the field of levosulpiride compound and its preparation, can solve the problems of low molar yield, long production cycle, and unstable products, etc., and achieve the goal of reducing production cost, increasing reaction yield, and shortening the production cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 20g of sulpiride, put it in an ultrasonic chemical reactor, add 82g of methanol, 16g of N,N-dimethylformamide, stir for 30 minutes, add 2g of ferric oxide fine powder, select the ultrasonic frequency of 32.45 kHz, keep for 2 hours, filter, Discard the filter residue, add 50 g of water, stir for 30 minutes, cool the filtrate to 2°C, select an ultrasonic frequency of 40kHz, vacuum degree of 0.02MPa, keep for 2 hours, crystallize, filter out, and vacuum dry at 50°C to obtain 21.38g of levosulpiride dihydrate , and the molar yield was 96.72%.
specific Embodiment 2
[0026] Take 20g of sulpiride, put it in an ultrasonic chemical reactor, add 84g of methanol, 18g of N,N-dimethylformamide, stir for 30 minutes, add 2g of ferric oxide fine powder, select the ultrasonic frequency of 32.45 kHz, keep for 2 hours, filter, Discard the filter residue, add 50g of water, stir for 30 minutes, cool the filtrate to 4°C, select an ultrasonic frequency of 50kHz, vacuum degree of 0.04MPa, keep it for 2 hours, crystallize, filter out, and vacuum dry at 60°C to obtain 21.47g of levosulpiride dihydrate , and the molar yield was 97.13%.
specific Embodiment 3
[0028] Take 20kg of sulpiride, put it in an ultrasonic chemical reactor, add 83kg of methanol, 17kg of N,N-dimethylformamide, stir for 30 minutes, add 2kg of ferric oxide fine powder, select the ultrasonic frequency of 32.45 kHz, keep for 2 hours, filter, Discard the filter residue, add 50kg of water, stir for 30 minutes, cool the filtrate to 3°C, select an ultrasonic frequency of 45kHz, vacuum degree of 0.03MPa, keep for 2 hours, crystallize, filter out, and vacuum dry at 55°C to obtain 21.66kg of levosulpiride dihydrate , and the molar yield was 98.0%.
[0029] The above examples illustrate that levosulpiride dihydrate can be made by adopting the extreme conditions and optimized conditions of the embodiment of the present invention, and the levosulpiride obtained in Example 3 is used to investigate the actual effect of the present invention below:
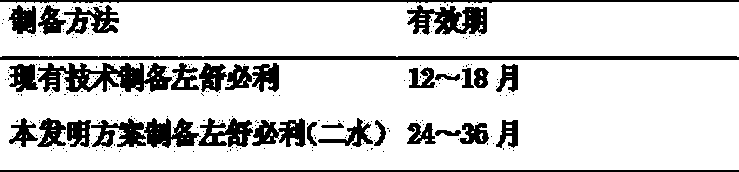
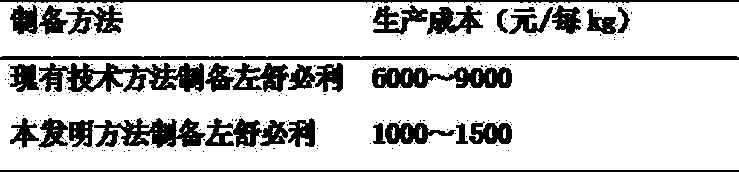
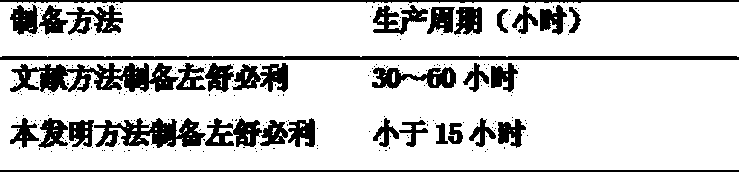
[0030] 1 The production cycle of levosulpiride prepared by the present invention is compared with the production cycle of levos...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com