Distyrene near ultraviolet photosensitizer comprising dihydroxyl-imino segment and synthesis method thereof
A stilbene-based, near-ultraviolet light technology, applied in the preparation of imino compounds, organic chemistry, etc., can solve the problems of rare near-ultraviolet photosensitizers, difficult to control the chemical composition of near-ultraviolet photosensitizers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 2,4-bis(2'-hydroxy-phenyl-methyl-imino)-stilbene:
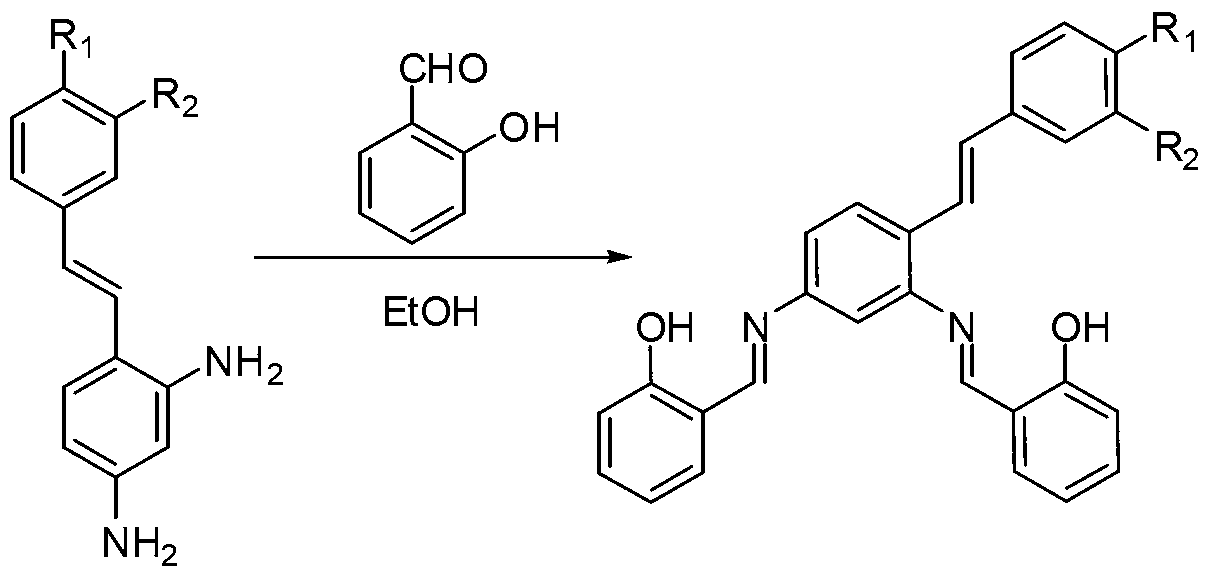
[0021] Mix 2,4-diamino-stilbene (5mmol, 1.05g) and 2-hydroxy-benzaldehyde (10mmol, 1.22g, 1.04ml) in a three-necked glass bottle, use 50ml ethanol as solvent, and stir magnetically at room temperature After 10 hours, the reaction was terminated, and the crude product was obtained by spin-drying under reduced pressure. Using cyclohexane as the eluent, the purified product was separated by silica gel chromatography. Yield: 75%, melting point: 162.6-163.8°C. That 1 The H NMR spectrum is as figure 1 as shown, 1 H-NMR (D 6 -DMSO,500MHz)δ(ppm):13.058(s,-OH,1H),13.005(s,-OH,1H),9.110(s,N=CH,1H),9.046(s,N=CH,1H ),7.945-7.928(d,Ar-H,1H),7.751-7.733(m,Ar-H,1H),7.686-7.668(m,Ar-H,1H),7.571-7.502(m,Ar-H , 4H), 7.485-7.393 (m, Ar-H, 5H), 7.363-7.280 (m, CH=CH, 2H), 7.053-6.986 (m, Ar-H, 4H).
[0022]
[0023] The preparation concentration is 1×10 -5 mol·l -1 The ethyl acetate solution of 2,4-bis(2'-hydroxy-phenyl-methyl...
Embodiment 2
[0025] 2,4-Bis(2’’-hydroxy-phenyl-methyl-imino)-4’-methoxy-stilbene
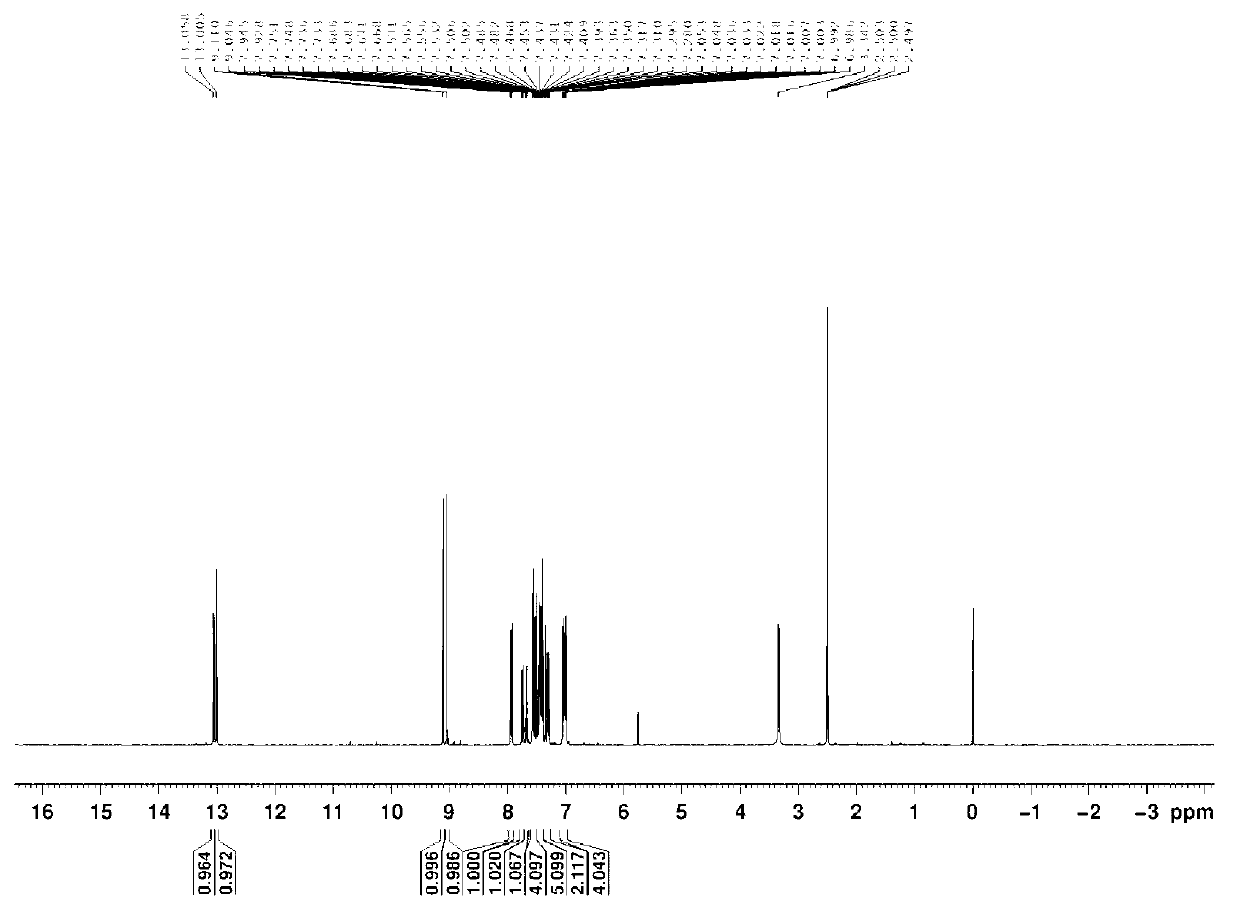
[0026] Mix 2,4-diamino-(4'-methoxy)-stilbene (5mmol, 1.05g) and 2-hydroxy-benzaldehyde (10mmol, 1.22g, 1.04ml) in a three-necked glass bottle, Using 50ml of ethanol as a solvent, magnetically stirred at room temperature for 10 hours to complete the reaction, spin-dried under reduced pressure to obtain a crude product, and used cyclohexane as an eluent to separate with a silica gel column to obtain a pure product. Yield: 75%, melting point: 137.0-139.0°C. That 1 The H NMR spectrum is as figure 2 as shown, 1 H-NMR (D 6 -DMSO,500MHz)δ(ppm):13.095(s,Ar-OH,1H),13.076(s,Ar-OH,1H),9.111(s,N=CH,1H),9.044(s,N=CH ,1H),7.912-7.896(d,Ar-H,1H),7.746-7.732(d,Ar-H,1H),7.682-7.669(d,Ar-H,1H),7.516-7.426(m,Ar -H,6H),7.412-7.226(m,CH=CH,2H),7.054-6.981(m,Ar-H,6H),3.784(s,O-CH 3 ,3H).
[0027]
[0028] The preparation concentration is 1×10 -5 mol·l -1 The ethyl acetate solution of 2,4-bis(2''-hydroxy-phenyl-methy...
Embodiment 3
[0030] 2,4-Bis(2’’-hydroxy-phenyl-methyl-imino)-3’,4’-dimethoxy-stilbene
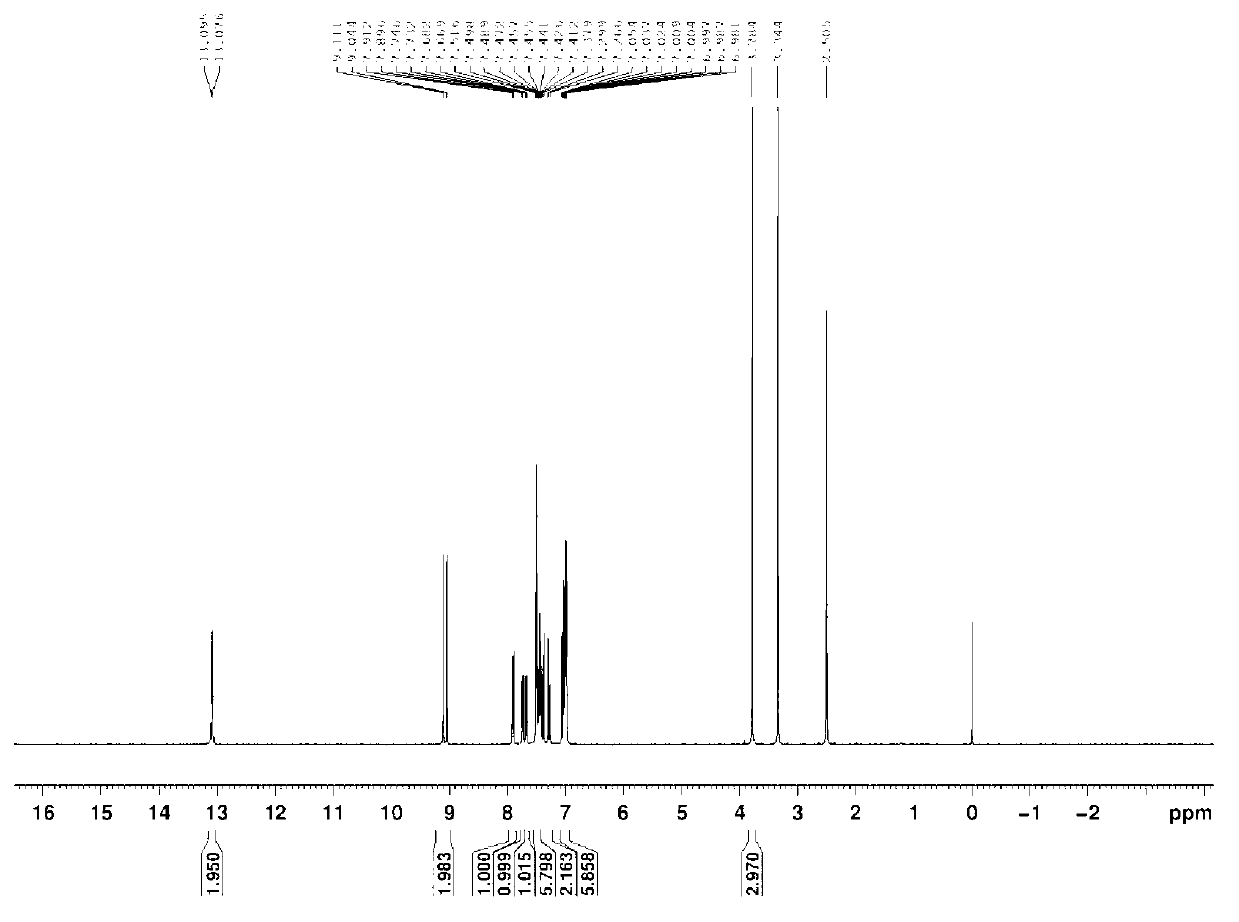
[0031] Put 2,4-diamino-(3',4'-dimethoxy)-stilbene (5mmol, 1.35g) and 2-hydroxy-benzaldehyde (10mmol, 1.22g, 1.04ml) in a three-mouth glass Mix in a bottle, use 50ml of ethanol as solvent, and magnetically stir at room temperature for 10 hours to end the reaction, spin dry under reduced pressure to obtain a crude product, use cyclohexane as an eluent, and separate with a silica gel chromatography column to obtain a pure product. Yield: 75%, melting point: 153.5-155.0°C. That 1 The H NMR spectrum is as image 3 as shown, 1 H-NMR (D 6 -DMSO,500MHz)δ(ppm):13.191(s,-OH,1H),13.101(s,-OH,1H),9.102(s,N=CH,1H),9.060(s,N=CH,1H ),7.893-7.876(d,Ar-H,1H),7.744-7.729(d,Ar-H,1H),7.681-7.666(d,Ar-H,1H),7.526-7.524(d,CH=CH ,1H),7.484-7.423(m,Ar-H,4H),7.269-7.236(d,Ar-H,1H),7.197-7.194(d,CH=CH,1H),7.107-7.090(d,Ar -H,1H),7.046-6.982(m,Ar-H,5H),3.827(s,O-CH 3 ,3H),3.782(s,O-CH 3 ,3H).
[0032]
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap