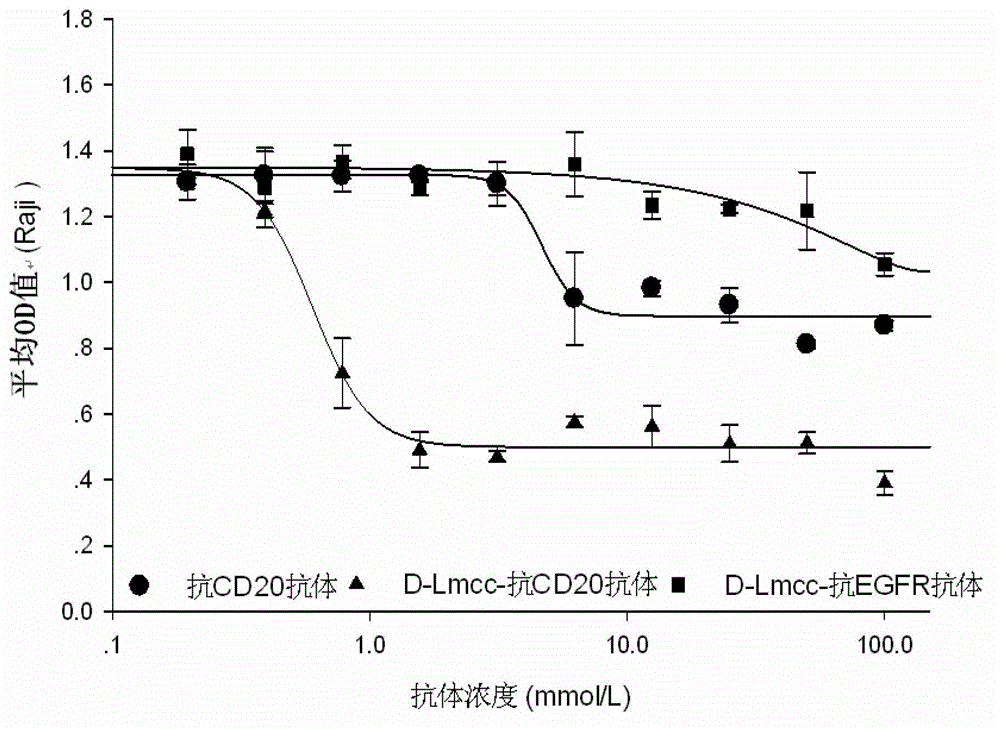
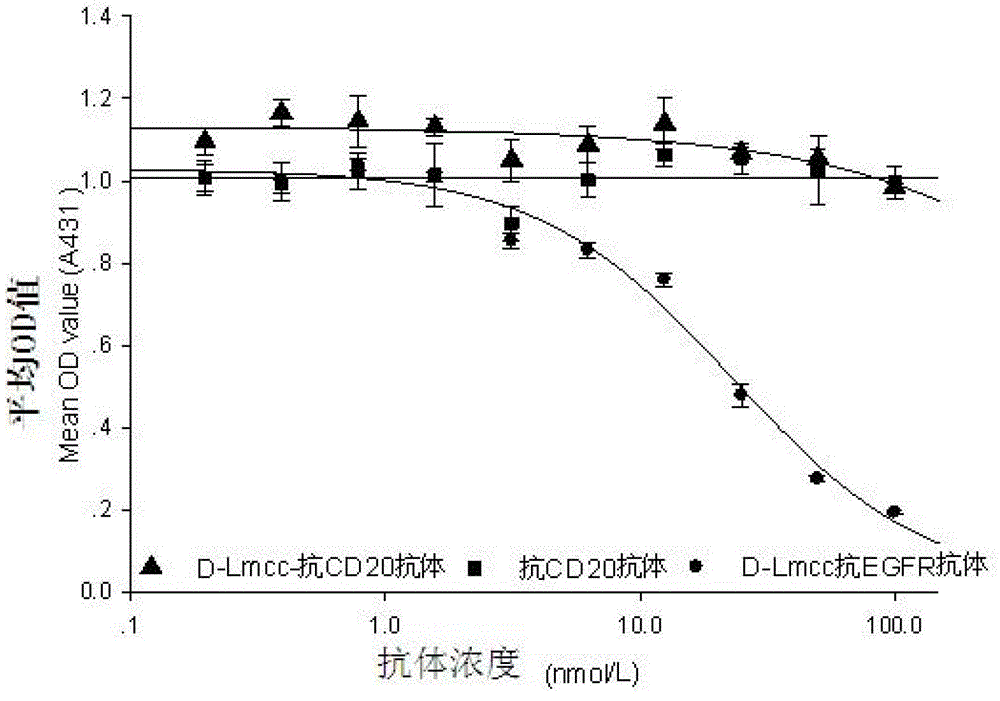
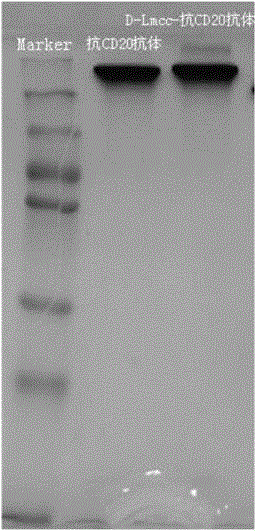
Anti-cell-acceptor and anti-tumor-growth drug molecule, preparation method thereof and applications thereof
A kind of use and pharmaceutical technology, applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, drug combination, pharmaceutical formulation, etc., can solve the problem of unsatisfactory toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] Esterification of maytansinol (MDC) with Fmoc-N-methyl-L-alanine (synthesis of Fmoc-N-Me-D / L-Ala-MDC)
[0250]
[0251] Weigh maytansinol (0.600g, 1.062mmol), Fmoc-N-methyl-L-alanine (6.911g, 21.24mmol), scandium trifluoromethanesulfonate (0.314g, 0.637mmol) and DMAP (0.389 g, 3.186mmol) was placed in a 250ml Schlenck bottle, dichloromethane (100mL) was added under nitrogen protection, and stirred at -8°C for 0.5 hours. Add DIC (2.949g, 23.37mmol) dropwise, continue to stir the reaction at -8°C for 0.5h, slowly warm up to room temperature, and recover the catalyst by filtration. The filtrate is quenched with dilute hydrochloric acid, extracted with dichloromethane, and then saturated Wash with sodium and saturated brine, dry over anhydrous sodium sulfate, and spin to dry the solvent. Column chromatography (silica gel, 300-400 mesh, CH 2 Cl 2 / MeOH 30:1) gave the diastereomeric mixture Fmoc-N-Me-D / L-Ala-MDC as a white solid (0.8385 g, 90.5% yield). Further column ...
Embodiment 2
[0253] Deprotection of Fmoc-N-Me-D / L-Ala-MDC (synthesis of N-Me-D / L-Ala-MDC)
[0254]
[0255] Dissolve Fmoc-N-Me-D / L-Ala-MDC (0.463g, 0.5307mmol) prepared in Example 1 in acetonitrile (200mL), add piperidine (0.865g, 10.15mmol), stir at room temperature for 4 hours, add water Quenching, extraction with dichloromethane, washing with saturated brine, drying over anhydrous sodium sulfate, rotary evaporation to remove the solvent, and drying to obtain a crude product. It was used in the next reaction without further purification. LC-MS (M+H + ) Calculated value: 650.3, measured value: 650.3. Rt: 3.96min.
Embodiment 3
[0257] Deprotection of Fmoc-N-Me-L-Ala-MDC (synthesis of N-Me-L-Ala-MDC)
[0258]
[0259] Fmoc-N-Me-L-Ala-MDC (0.463g, 0.5307mmol) prepared in Example 1 was dissolved in acetonitrile (200mL), piperidine (0.865g, 10.15mmol) was added, stirred at room temperature for 4 hours, and quenched with water , extracted with dichloromethane, washed with saturated brine, dried over anhydrous sodium sulfate, removed the solvent by rotary evaporation, and dried to obtain a crude product. It was used in the next reaction without further purification. LC-MS (M+H + ) calculated value: 650.3, measured value: 650.3. Rt: 3.96min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com