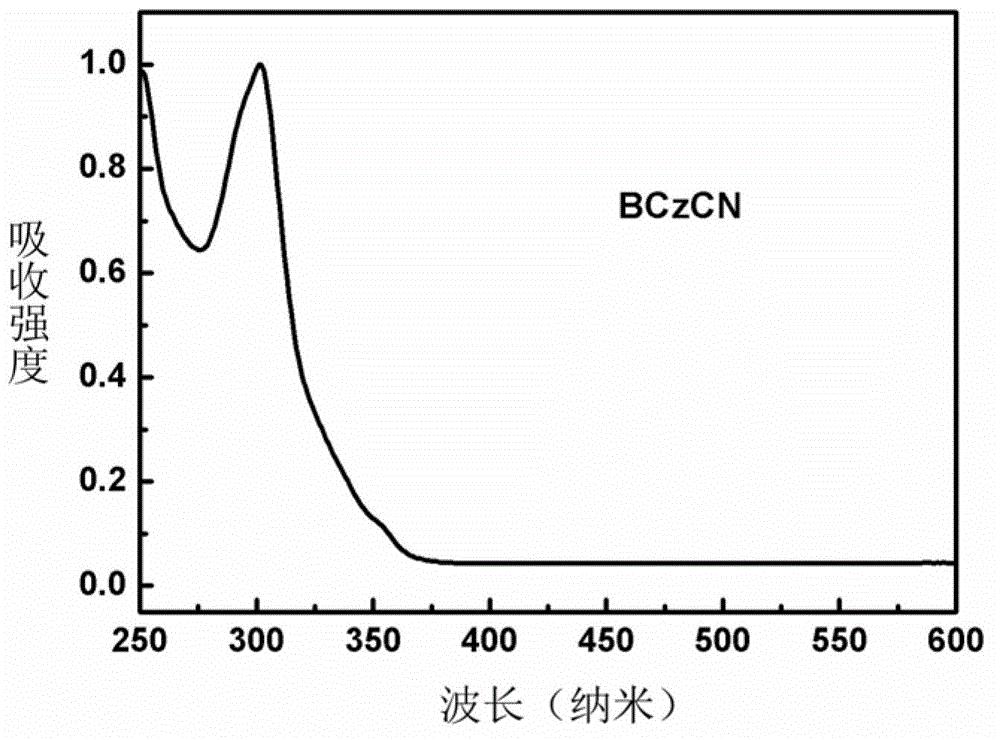
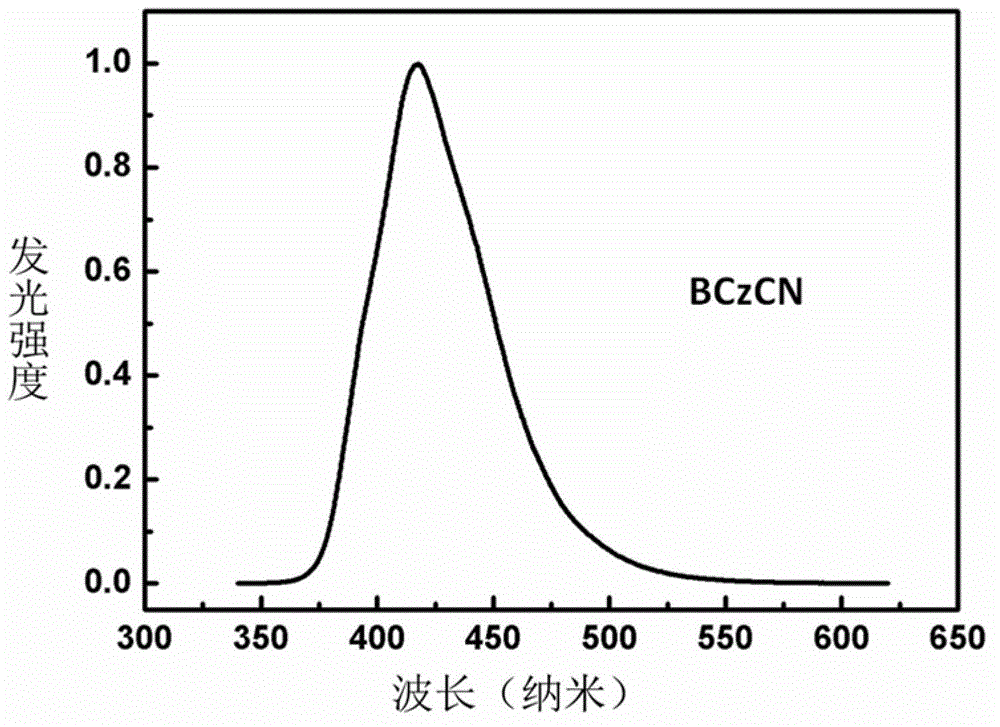
N-phenylcarbazole derivative and application thereof to electrophosphorescent device
A technology of phenylcarbazole and phosphorescent devices is applied in the application field of blue phosphorescent host materials in organic electrophosphorescent devices, and can solve the problems of low glass transition temperature, hindering wide application and low material stability, etc. The effect of device performance improvement, conjugation reduction, and high-efficiency electroluminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Step 1: Add 4.00 grams of 3,6-dibromocarbazole and 3.20 grams of N-phenylcarbazole-3-boronic acid into a 50-ml flask, add catalyst Pd (PPh 3 ) 4 650mg, THF 42ml, 2M K 2 CO 3 The solution was 14 ml, refluxed under argon protection for 24 hours, extracted with dichloromethane after cooling, the organic layer was dried with anhydrous sodium sulfate and then spin-dried, passed through the column with dichloromethane / petroleum ether=1:5 (volume ratio), and spin-dried 4.70 g of 6-bromo-N,N-diphenyl-3,3'-biscarbazole were obtained by drying, with a yield of 83.7%.
[0038] Step 2: Add 4.0 grams of 6-bromo-N,N-diphenyl-3,3'-biscarbazole and 4.0 grams of cuprous cyanide into a 50 ml flask, add N,N-dimethylformamide 30 ml, reflux under argon protection for 24 hours, extract with dichloromethane after cooling, dry the organic layer with anhydrous sodium sulfate and spin dry, pass through the column with dichloromethane / petroleum ether=1:5 (volume ratio), spin dry 2.91 g of 6-c...
Embodiment 2
[0040] Step 1: 4.00 grams of 3-bromocarbazole and 3.20 grams of N-phenylcarbazole-3-boronic acid are added in a 50 ml flask, and catalyst Pd(PPh 3 ) 4 650mg, THF 42ml, 2M K 2 CO 3The solution was 14 ml, refluxed under argon protection for 24 hours, extracted with dichloromethane after cooling, the organic layer was dried with anhydrous sodium sulfate and then spin-dried, passed through the column with dichloromethane / petroleum ether=1:5 (volume ratio), and spin-dried 5.33 g of N,N-diphenyl-3,3'-biscarbazole were obtained by drying, and the yield was 88.6%.
[0041] Step 2: Add 4.00 g of N,N-diphenyl-3,3'-biscarbazole and 30 ml of N,N-dimethylformamide into a 50 ml flask, cool to about 0°C in an ice bath, and separate Add 4.00 g of 1,3-dibromo-5,5-dimethylhydantoin in batches, and keep the temperature below 5°C for 8 hours. The reaction mixture was poured into water, extracted with dichloromethane, washed with water, washed with saturated brine, dried with anhydrous sodium ...
Embodiment 3
[0044] Step 1: Add 4.00 grams of 1,3-dibromobenzene and 3.20 grams of N-phenylcarbazole-3-boronic acid into a 50-ml flask, add catalyst Pd(PPh 3 ) 4 650mg, THF 42ml, 2M K 2 CO 3 The solution was 14 ml, refluxed under argon protection for 24 hours, extracted with dichloromethane after cooling, the organic layer was dried with anhydrous sodium sulfate and then spin-dried, passed through the column with dichloromethane / petroleum ether=1:5 (volume ratio), and spin-dried 7.44 g of 1,3-bis(N-phenylcarbazol-3-yl)benzene were obtained by drying. The yield was 78.3%.
[0045] Step 2: Add 4.00 g of 1,3-bis(N-phenylcarbazol-3-yl)benzene and 30 ml of N,N-dimethylformamide into a 50 ml flask, cool in an ice bath to about 0°C, Add 4.00 g of 1,3-dibromo-5,5-dimethylhydantoin in batches, and keep the temperature below 0°C for 8 hours. The reaction mixture was poured into water, extracted with dichloromethane, washed with water, washed with saturated brine, dried with anhydrous sodium sulf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com