Primer pair and probe for detecting avian influenza virus in sample by fluorescence RT-PCR and kit containing primer pair and probe
A technology for RT-PCR and avian influenza virus, applied in the field of kits containing the primer and probe sequences, can solve the problems of unsuitable virus early diagnosis, PCR product contamination, non-specific amplification, etc., and shorten the detection cycle , high-throughput detection, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Primer and probe design, establishment and optimization of reaction system
[0061] 1. Primer and probe design:
[0062] Through comparative analysis of all known avian influenza virus H7 (including H7N9) genome sequences, select highly conserved segments with no secondary structure, and design multiple pairs of primers and probes. The length of the primers is generally 20 bases Left and right, there is no complementary sequence between and within the primers. The optimal primer and probe sequence combinations are as follows:
[0063] Upstream primer H7N9-4.0-F: GGTTTAGCTTCGGGGCATC
[0064] Downstream primer H7N9-4.0-R: TATATACAAATAGTGCACCGCATGT
[0065] Probe H7N9-4.0-B: TTATGCAAATGAAAACCAATCCCATTG
[0066] 2. Establishment and optimization of the reaction system:
[0067] The target region template is obtained by the following method: use the inactivated H7 strain of avian influenza virus (including H7N9, provided by China Institute for the Control of...
Embodiment 2
[0082] Example 2 Sensitivity experiment for detecting the copy number of H7N9 subtype influenza virus
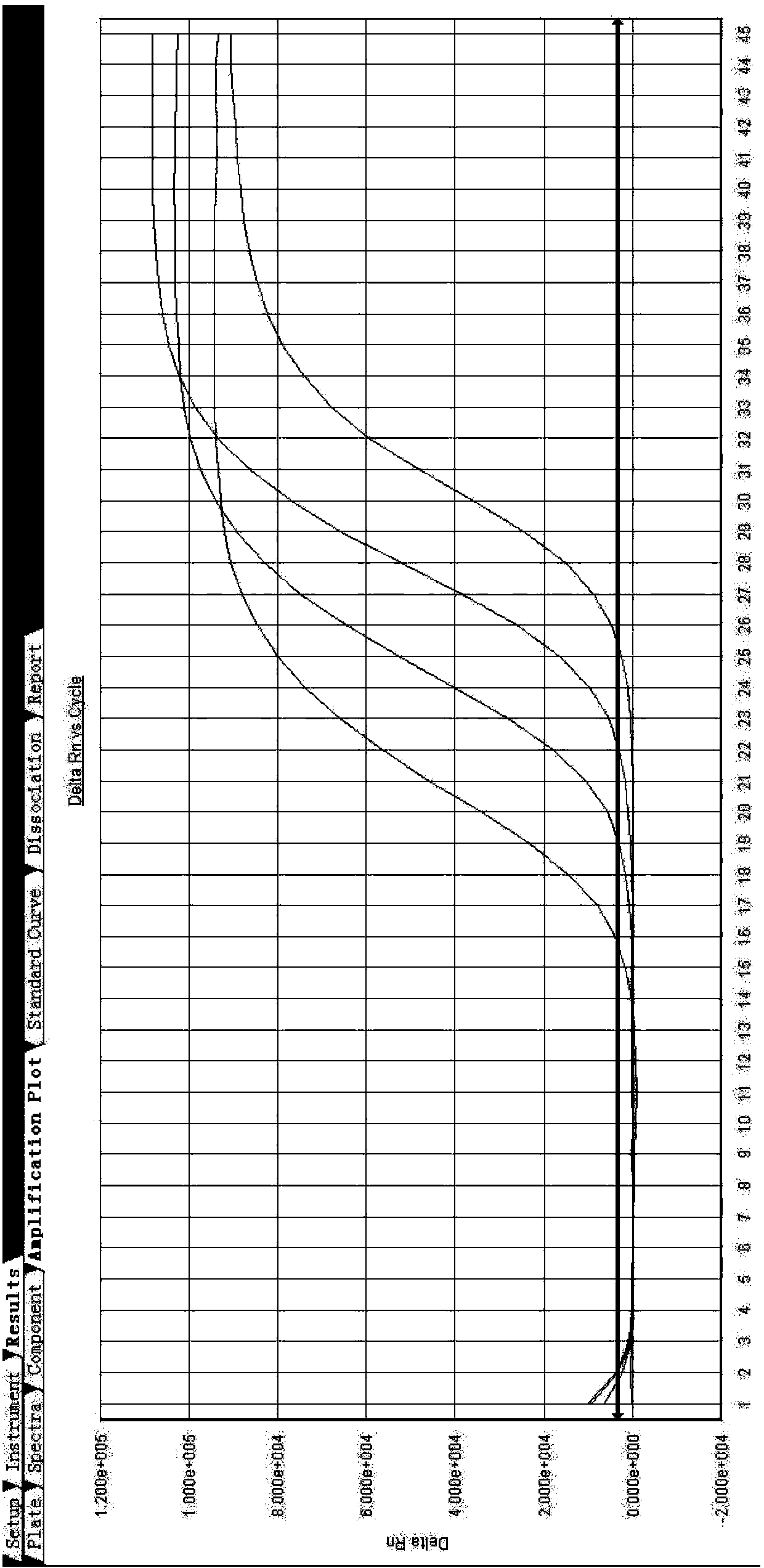
[0083] Set the concentration to 10 6 copies / ml of the H7N9 inactivated strain provided by China Biological Products Inspection Institute, diluted to 10 by 10 times 5 copies / ml, 10 4 copies / ml, 10 3 copies / ml, 10 2 copies / ml, using the primers, probes, and established reaction systems, instruments, and amplification conditions designed in steps 1-4 in Example 1, to carry out fluorescent RT-PCR detection.
[0084] The results showed that in 10 3 copies / ml, the CT value is 25, and other indicators also meet the detection requirements. Therefore, the present invention improves the sensitivity of detection by optimizing primers and probes, and its sensitivity can reach 10 3 copies / ml. see results figure 1 .
Embodiment 3
[0085] Example 3 A Specific Experiment for Detecting H7N9 Subtype Influenza Virus
[0086] This experimental example proves that the reagents or kits provided by the present invention can detect viruses in various positive samples under established conditions, and negative samples have no detection results.
[0087] 1. Sample:
[0088] 120 samples were tested, including 31 positive samples determined by the method recommended by the Chinese Center for Disease Control and Prevention to be positive for avian influenza virus H7N9; the other 89 were negative samples. These 120 samples were throat swabs. Sample processing: Add 500 μl of PBS or saline to the centrifuge tube containing the swab, shake vigorously for 30 sec. Squeeze out the liquid as much as possible and move it to a centrifuge tube for 20 sec at 6000rpm, and take the supernatant for later use.
[0089] Negative control: DEPC water;
[0090] Positive control: H7N9 inactivated strain, provided by China Institute ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com