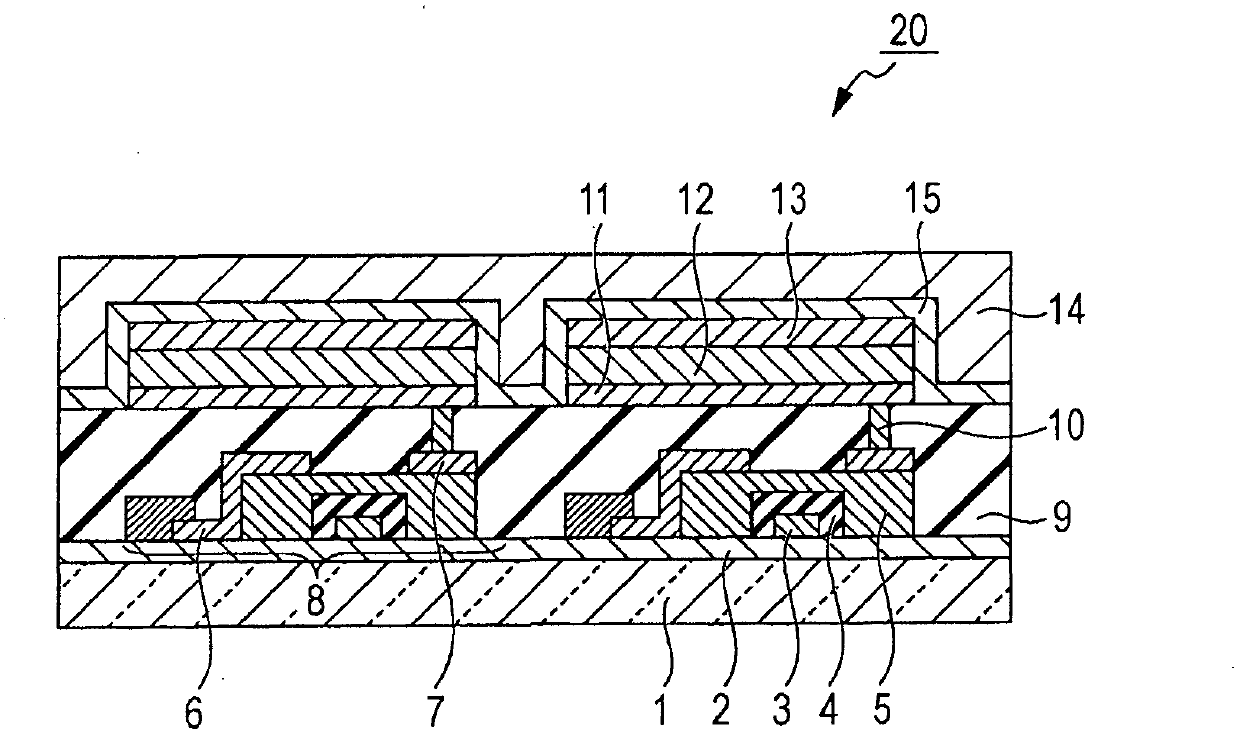
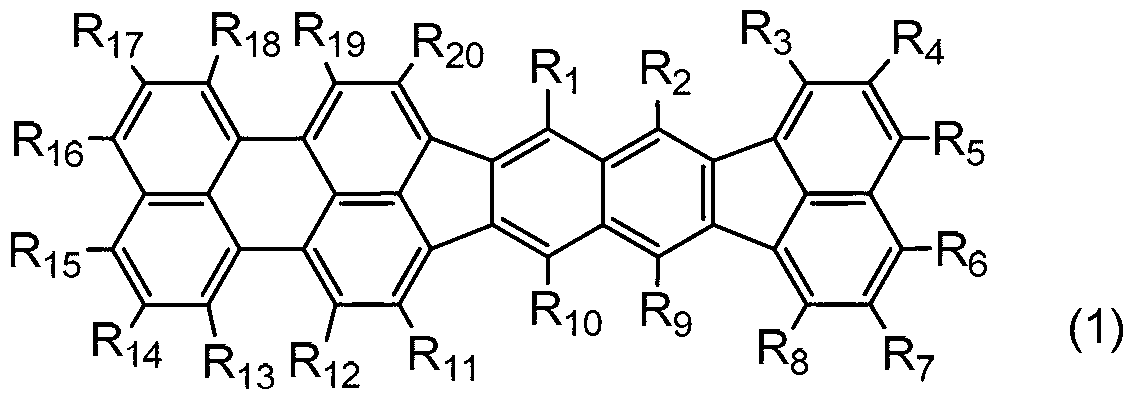
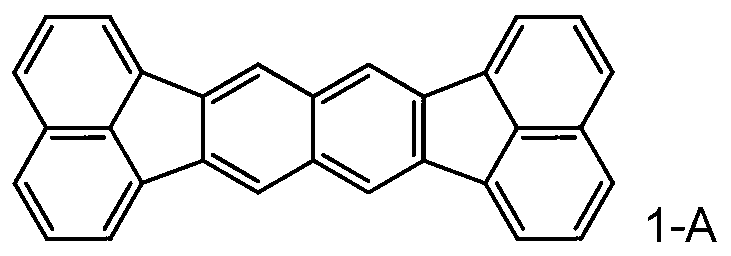
Organic compound, organic light-emitting device, and image display device
A technology of organic light-emitting devices and organic compounds, applied in the fields of organic semiconductor devices, organic chemistry, chemical instruments and methods, etc., can solve the problems of unsatisfactory development and achieve good sublimation and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] The synthesis of exemplary compound XX-1
[0110]
[0111] (1) Synthesis of compound X3
[0112] The following reactants and solvent were placed in the reactor.
[0113] Compound X1: 4.0 g (13 mmol)
[0114] Compound X2: 2.8g (13mmol)
[0115] Ethanol: 65ml
[0116] Next, the reaction solution was heated to 60° C., and then 10 ml of a 6M aqueous sodium hydroxide solution was added dropwise to the solution. After the addition was complete, the reaction solution was heated to 80°C and stirred at this temperature (80°C) for 2 hours. Next, the reaction solution was cooled to generate a precipitate, which was then filtered off. Next, the obtained precipitate was sequentially washed with water and ethanol, and then dried by heating at 80° C. under reduced pressure to prepare 6 g of Compound X3 as a dark green solid (yield: 89%).
[0117] (2) Synthesis of compound X5
[0118] The following reactants and solvent were placed in the reactor.
[0119] Compound X3: 5.0 g...
Embodiment 2
[0137] The synthesis of exemplary compound XX-2
[0138]
[0139] (1) Synthesis of Compound X8
[0140] The following reactants and solvent were placed in the reactor.
[0141] Compound X7: 10g (55mmol)
[0142] Compound X2: 12g (55mmol)
[0143] Ethanol: 200ml
[0144] Next, the reaction solution was heated to 60° C., and then 20 ml of a 6M aqueous sodium hydroxide solution was added dropwise to the solution. After the addition was complete, the reaction solution was heated to 80°C and stirred at this temperature (80°C) for 2 hours. Next, the reaction solution was cooled to generate a precipitate, which was then filtered off. Next, the obtained precipitate was sequentially washed with water and ethanol, and then dried by heating at 80° C. under reduced pressure to prepare 18 g of Compound X8 as a dark green solid (yield: 92%).
[0145] (2) Synthesis of compound X9
[0146] The following reactants and solvent were placed in the reactor.
[0147] Compound X8: 10g (28...
Embodiment 3
[0165] The synthesis of exemplary compound XX-5
[0166] Exemplary compound XX-5 was prepared by synthesis according to the same method as in Example 2, except that in Example 2(1), Compound X11 shown below was used instead of Compound X2.
[0167]
[0168] As a result of measuring the purity of the obtained compound by HPLC, it was confirmed that the purity was 99.5% or more.
[0169] In addition, the toluene solution of the exemplary compound XX-5 (1×10 -5 mol / L) emission spectrum. In this embodiment, the excitation wavelength is 500 nm. As a result, an emission spectrum having a peak intensity at 550 nm was obtained.
[0170] Furthermore, the compound was identified by measuring the molecular weight using JMS-T100TD (DART-TOF-MASS) manufactured by JEOL, Ltd.
[0171] DART-TOF-MASS: M+H=805.3
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com