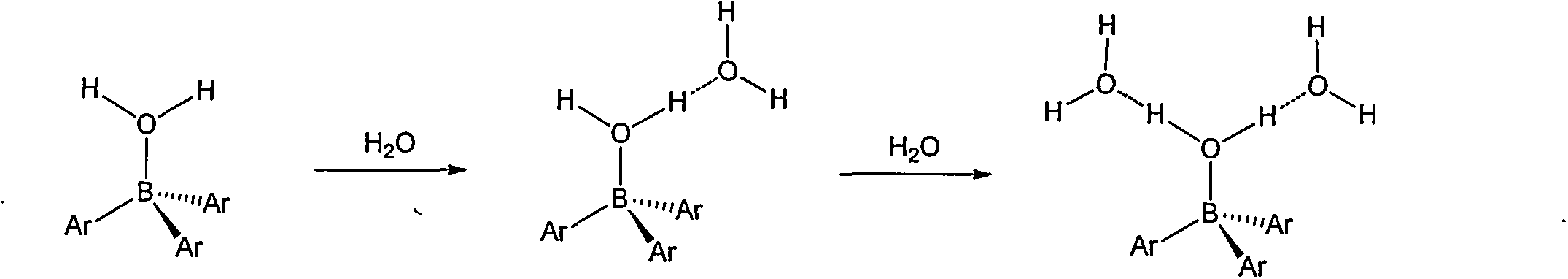
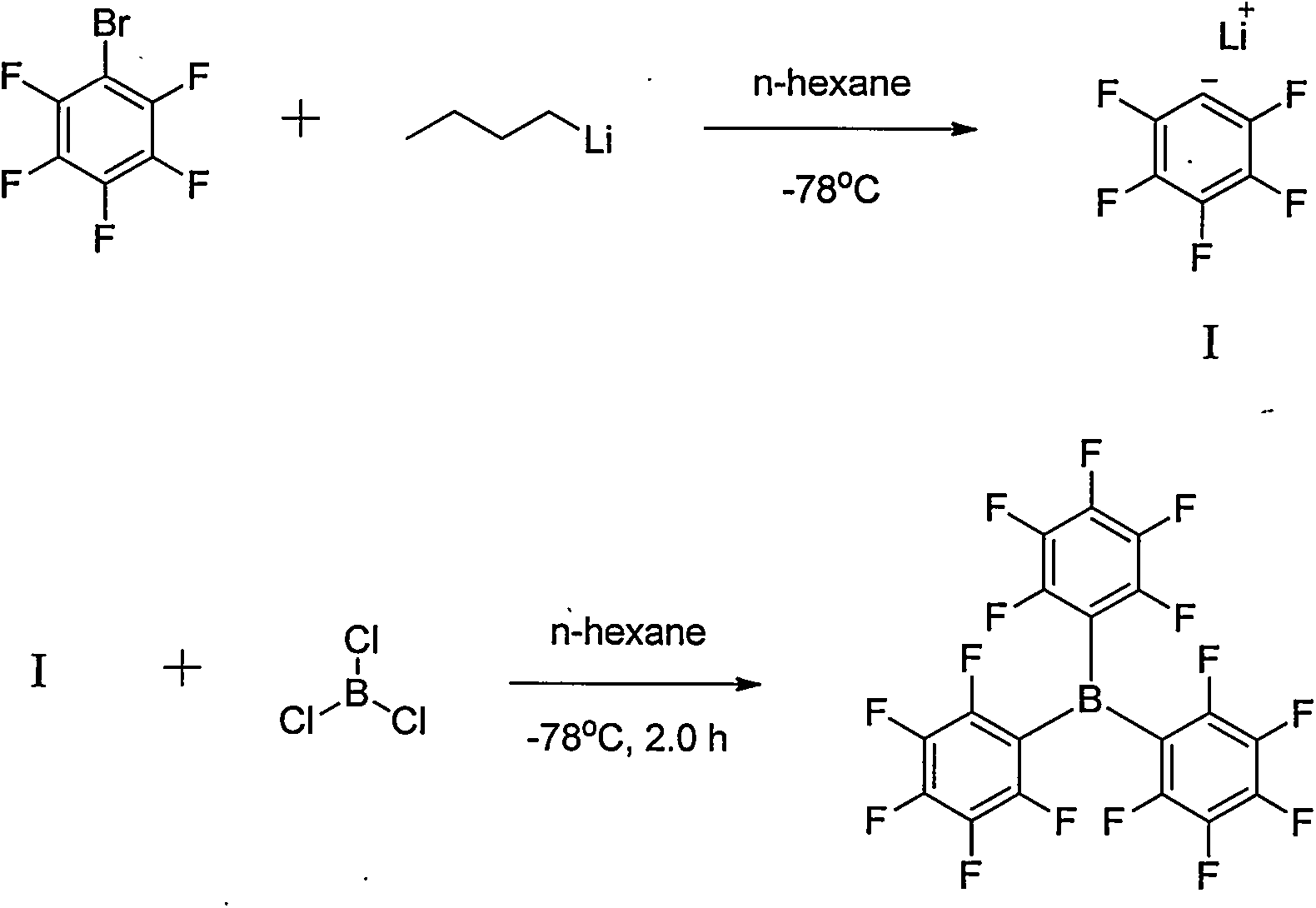
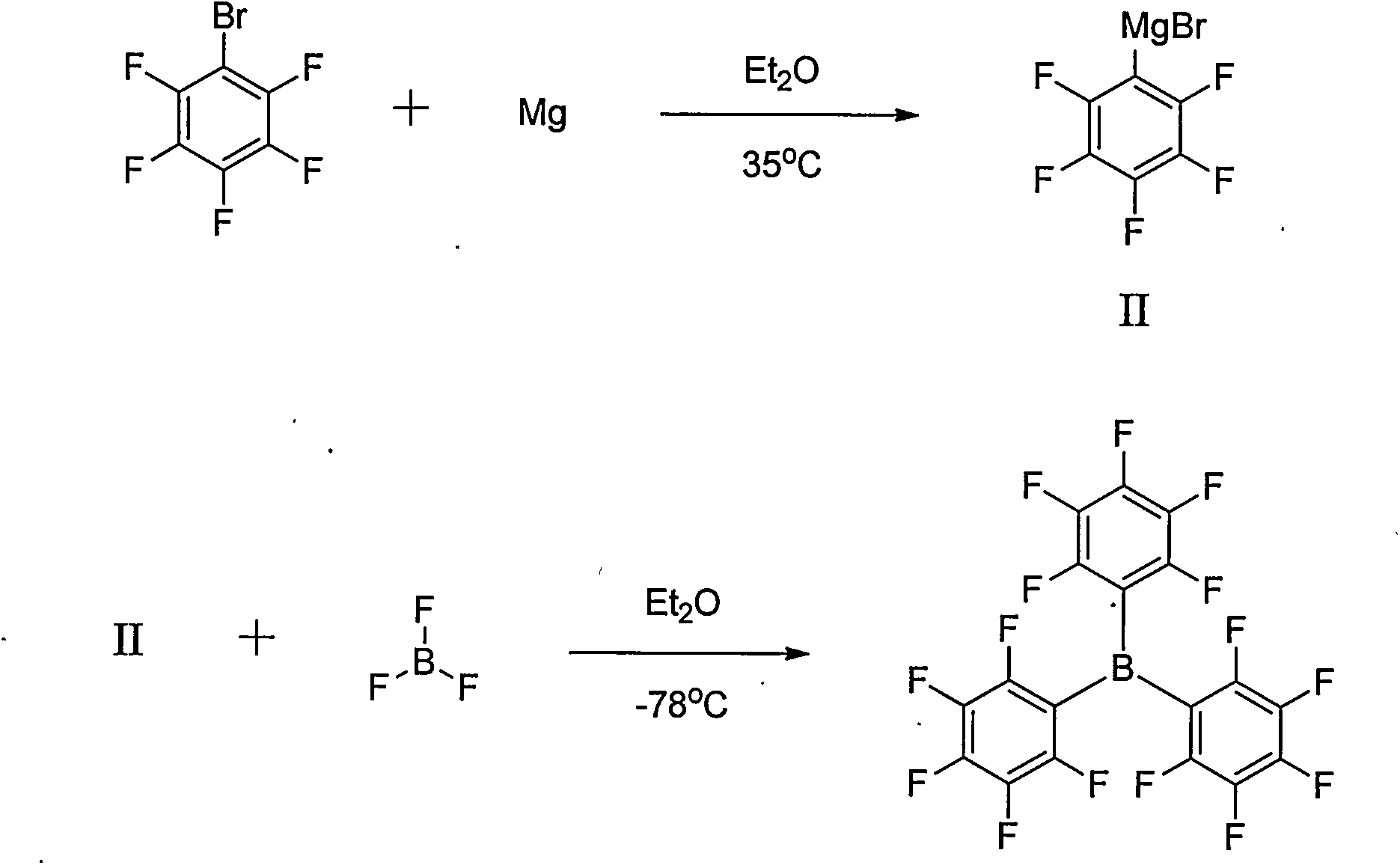
Preparation method of tri(pentafluorophenyl) borane
A kind of technology of pentafluorophenyl and pentafluorobromobenzene, applied in the field of preparation of triborane, can solve problems such as high price, application has not been paid enough attention, etc., and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] After adding pentafluorobromobenzene (0.09mol, 22.2g) into a three-necked flask (250mL), 2-methyltetrahydrofuran (100mL) was added. At 25°C, n-BuLi (2.5M, 0.09mol, 36mL) was added dropwise. After reacting for 6.0h, trimethyl borate (0.03mol, 3.1g) was added dropwise at 25°C for 10min. After 16 h at 25°C, the reaction was monitored by GC. Pour the reaction solution into water, extract twice with 2-methyltetrahydrofuran, combine the organic layers, wash twice with water and once with saturated brine, dry the organic layer with sodium sulfate, evaporate 2-methyltetrahydrofuran under reduced pressure, and recover 2-Methyltetrahydrofuran was obtained as a crude product, which was recrystallized from n-hexane to obtain tris(pentafluorophenyl)borane (0.0049mol, 2.48g), with a yield of 16.2%.
Embodiment 2
[0027] After adding pentafluorobromobenzene (0.09mol, 22.2g) into a three-necked flask (250mL), 2-methyltetrahydrofuran (100mL) was added. EtMgBr (1.0M, 0.09mol, 90mL) was added dropwise at 25°C. After reacting for 6.0h, trimethyl borate (0.03mol, 3.1g) was added dropwise at 25°C for 10min. After 16 h at 25°C, the reaction was monitored by GC. Pour the reaction solution into water, extract twice with 2-methyltetrahydrofuran, combine the organic layers, wash twice with water and once with saturated brine, dry the organic layer with sodium sulfate, evaporate 2-methyltetrahydrofuran under reduced pressure, and recover 2-Methyltetrahydrofuran was obtained as a crude product, which was recrystallized from n-hexane to obtain tris(pentafluorophenyl)borane (0.0125 mol, 6.40 g), with a yield of 41.7%.
Embodiment 3
[0029] After adding pentafluorobromobenzene (0.09mol, 22.2g) into a three-necked flask (250mL), diethyl ether (100mL) was added. At 25°C, i-PrMgCl (2.0M, 0.09mol, 45mL) was added dropwise. After reacting for 6.0h, trimethyl borate (0.03mol, 3.1g) was added dropwise at 25°C for 10min. After 16 h at 25°C, the reaction was monitored by GC. Pour the reaction solution into water, extract twice with 2-methyltetrahydrofuran, combine the organic layers, wash twice with water and once with saturated brine, dry the organic layer with sodium sulfate, evaporate 2-methyltetrahydrofuran under reduced pressure, and recover 2-Methyltetrahydrofuran was obtained as a crude product, which was recrystallized from n-hexane to obtain tris(pentafluorophenyl)borane (0.0182mol, 9.28g), with a yield of 60.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com