Uses of cimiracemate and related compounds for treating inflammation and modulating immune responses
A compound, lipoxygenase technology, used in anti-inflammatory agents, drug combinations, plant raw materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0149] Preparation of cell extracts
[0150] To collect whole-cell lysates, wash PBMac with cold PBS and dissolve in cold lysis buffer (50 mM tris(hydroxymethyl)aminomethane hydrochloride (Tris-C1) (pH 7.4); 150 mM chloride Sodium (NaCl); 50 mM Sodium Fluoride (NaF); 10 mM β-Glycerol Phosphate; 0.1 mM Ethylenediaminetetraacetic Acid (EDTA); 10% Glycerol; 1% Triton X-100; 1 mM Benzyl sulfonyl fluoride (PMSF); 1 mM sodium orthovanadate; 2 μg / ml pepstatin A; 2 μg / ml aprotinin; and 2 μg / ml leupeptin) for 20 min. Then, the lysate was centrifuged at 4°C for 20 minutes. Collect the supernatant and store it at -70 °C until use.
[0151] To collect nuclear protein extracts, treated cells were washed with PBS and resuspended in buffer A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.9), 10 mM potassium chloride (KCl), 0.1 mM EDTA, 0.1 mM ethylene glycol tetraacetic acid (EGTA). 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride or phenylmethylsul...
Embodiment 1
[0161] Example 1 - Prediction of Cimiracemate A as a Lipoxygenase Inhibitor
[0162] Similarity Ensemble (SEA), a search tool provided by Shoichet's lab in the Department of Medicinal Chemistry at the University of California, San Francisco (UCSF), quantitatively grouped Target protein pharmacology grouping and correlating target protein pharmacology 1-10 . Similarity values between ligands are expressed as expected values (E-values), which can be used to supplement chemical similarity values generated by BLAST 1,10-11 .
[0163] A SEA search was performed using cimi A as a query compound to generate an E-value between cimi A and the ligand of the target protein. The Tanimoto coefficient (Tc) for chemical similarity was also calculated. E-value less than 1×10 -10 Indicates a significant similarity, while an E-value greater than 1.0 indicates an insignificant similarity. A Tc value between 0 and 0.5 indicates an insignificant similarity, while a Tc greater than 0.5 ...
Embodiment 2
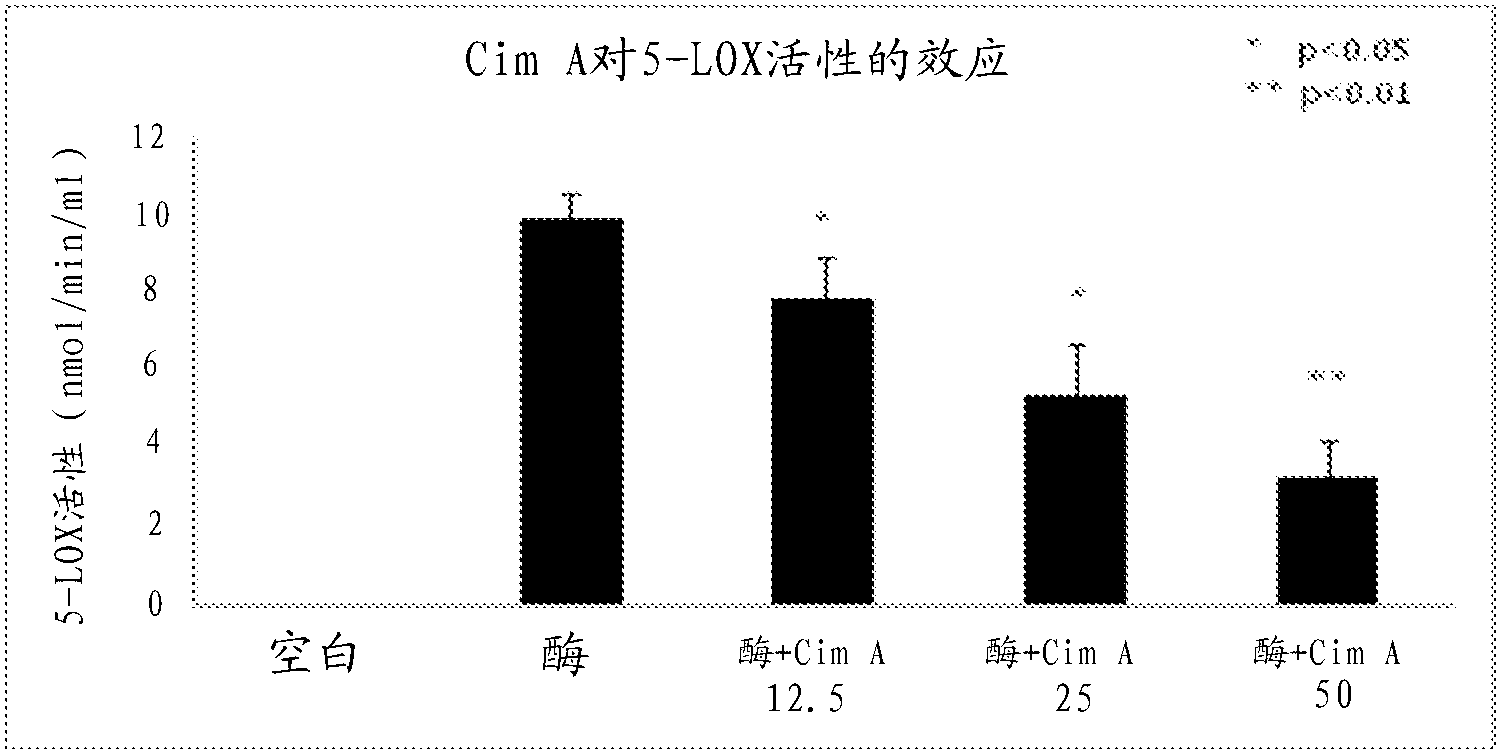
[0166] Example 2—Determination of the inhibitory effect of cimiracemate A on 5-lipoxygenase activity
[0167] This example demonstrates that cimiracemate A strongly inhibits the activity of 5-LOX. Briefly, 5-LOX inhibition assays were performed by using Cayman's Lipoxygenase Inhibitor Screening Assay Kit (Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer's instructions. 5-LOX and linoleic acid (substrate) were simultaneously added to dimethyl sulfoxide (DMSO) (control) or cimiracemate A at concentrations of 12.5, 25 and 50 μg / ml, respectively. After 5 min of incubation, the developer for the 5-LOX inhibition assay was added. Hydroperoxide production was measured with a microplate reader at a wavelength of 490 nm. The enzyme activity of 5-LOX is calculated in nmol / min / ml. The results showed that cimiracemate strongly inhibited 5-LOX activity in a dose-dependent manner (Fig. 1A).
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com