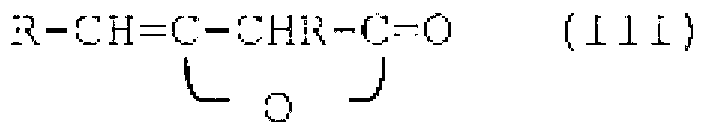Compositions comprising quat compounds and organopolysiloxanes
A technology of polysiloxane and siloxane copolymer, which is applied in the directions of organic cleaning compositions, softening compositions, detergent compositions, etc., can solve the problems of complex synthesis sequence and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
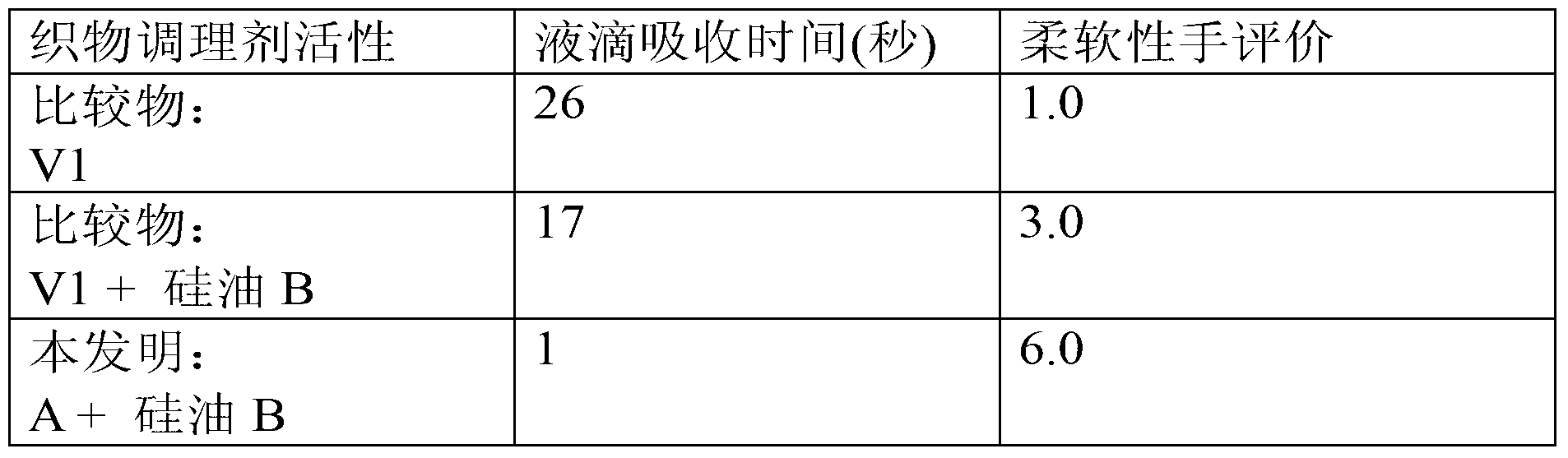
Examples
Embodiment 1
[0194] 119 g of an alkyl-ketene dimer (alkyl R about 16 carbon atoms, commercially available as "AKD" from Trigon Chemie GmbH) having a diketene equivalent of 570 g / mole were carefully melted without overheating . At 63°C, with cooling and efficient stirring, 21.4 g of 3-dimethylaminopropylamine were gradually metered in so that the internal temperature of 75°C was not exceeded. A few minutes after the spray addition was complete, the amidation was complete. The amidoamine was obtained with an amine number of 1.48 (theoretical 1.49), indicating practically 100% yield.
[0195] The amidoamine obtained is heated to 100° C. and a total of 37.2 g of methyl p-toluenesulfonate (0.95 moles per mole of tertiary amino groups in the amidoamine) are added within a period of 30 minutes. The system was reacted for an additional 2 hours at 100° C. to obtain 177.6 g of dialkylacetoacetamidoquaternary ammonium, which solidified on cooling. The product has an amine number of 0.07, which cor...
Embodiment 2
[0197] 119 g of the alkyl-ketene dimer described in Example 1 (570 g / mole of diketene) was mixed with a total of 39.2 g of bis(3-dimethylaminopropyl)amine (under the trade name "JEFFCAT Z130" commercially available from Huntsman) was gradually mixed. Shortly after capping the sparged end, the exothermic amidation reaction was complete. Afterwards, the amidoamine obtained was mixed with 74.5 g of methyl p-toluenesulfonate (0.95 mol per mole of tertiary amino groups in the amidoamine) at 100° C., added a little at a time, while the reaction mixture reached 118° C. . The reaction was allowed to complete at 100°C over the course of 2 hours. Based on an amine number of 0.11, about 94% of the tertiary amino groups are quaternized.
[0198] Production of emulsion (A) from the β-ketocarbonyl quaternary ammoniums of the invention:
[0199] 5.0 g of the β-ketocarbonyl quaternary ammonium (AKD Quat1) obtained in the examples were carefully melted in a glass beaker and mixed with 0....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com