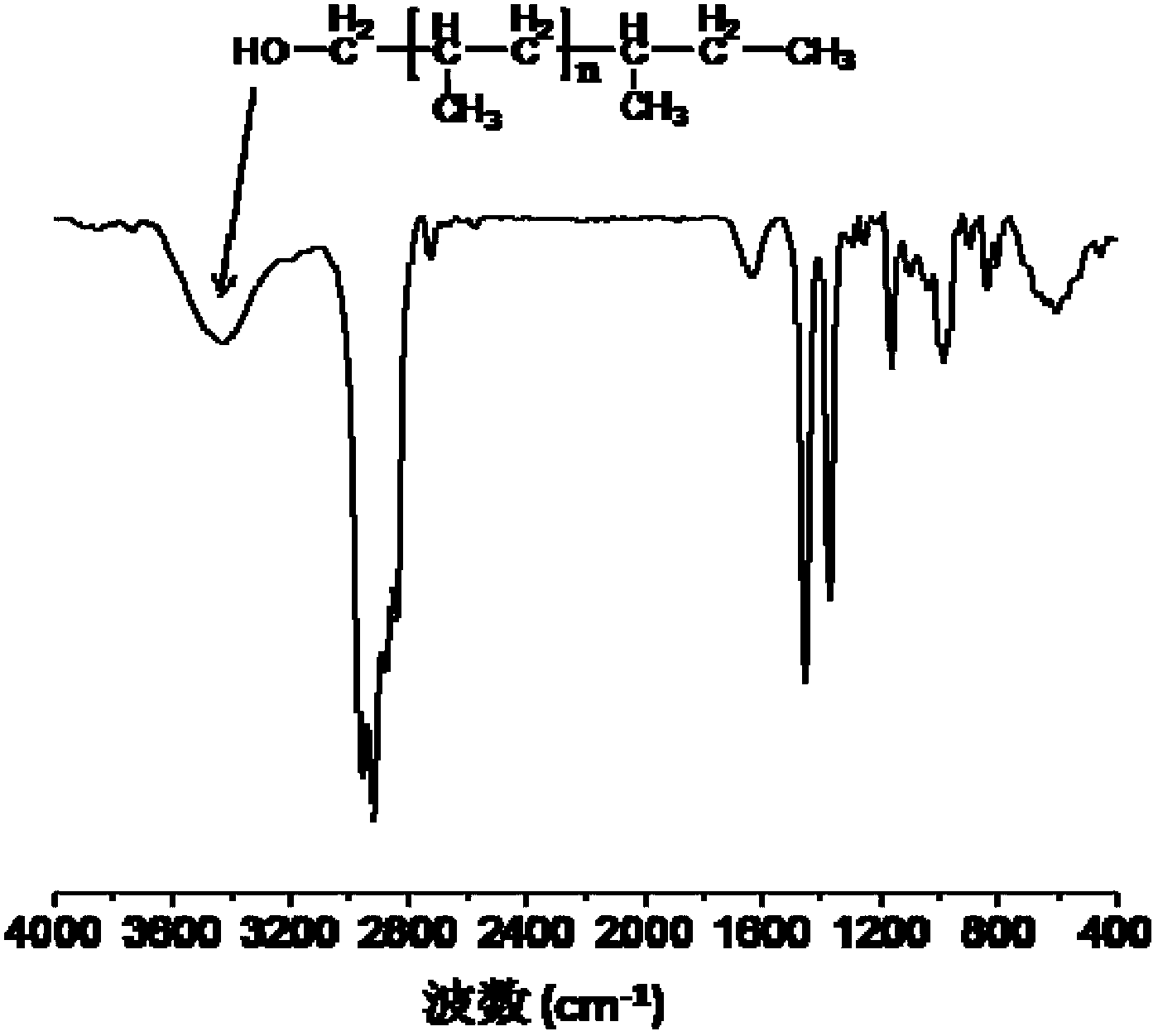
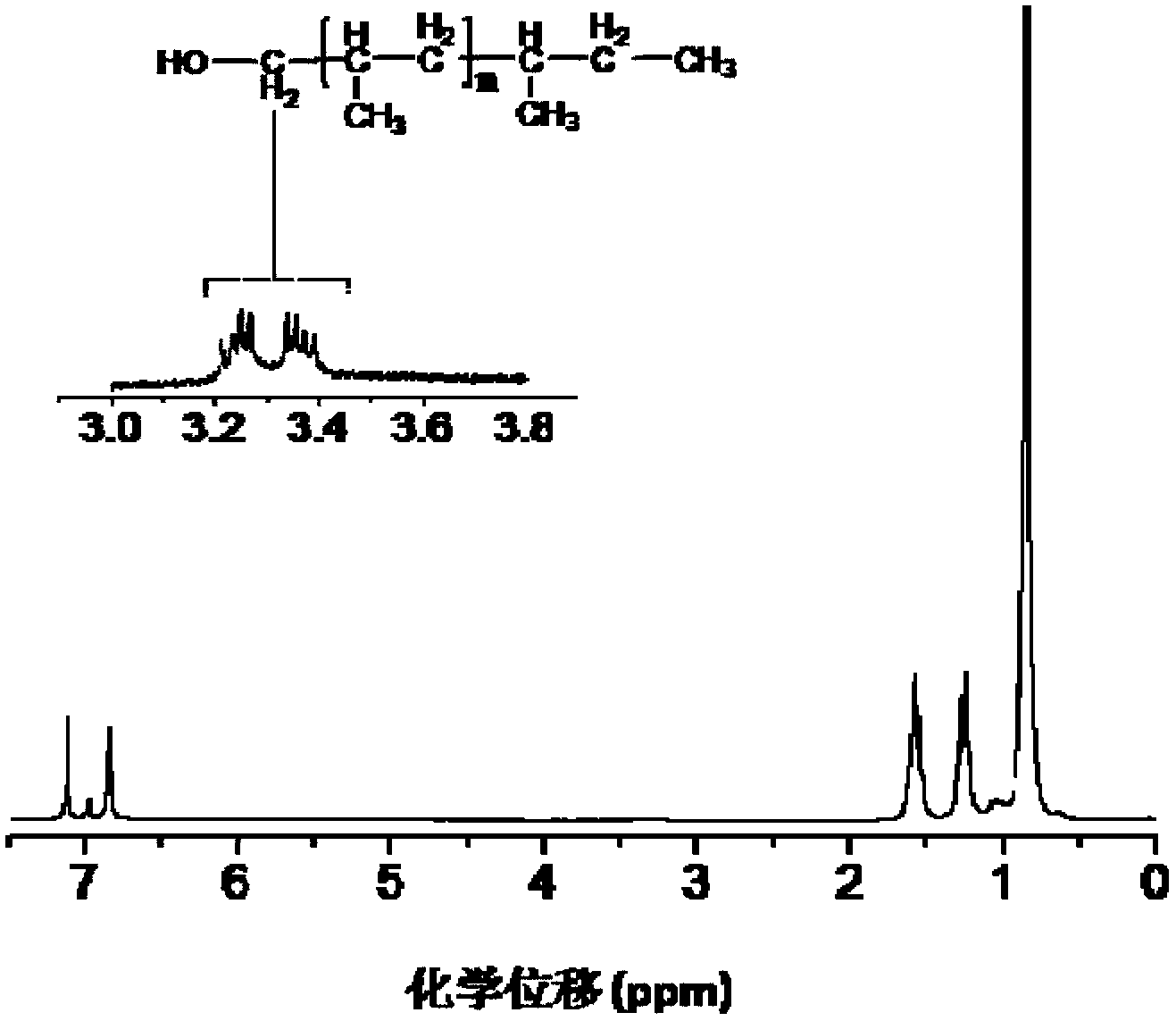
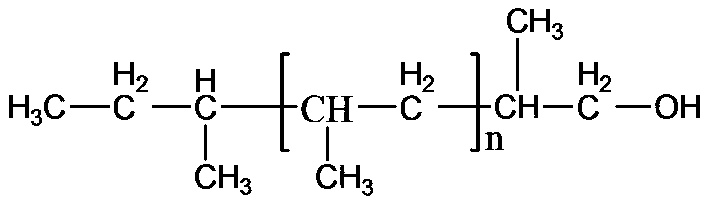
Isotatic polypropylene with functionalized terminal hydroxyl group and preparation method of isotatic polypropylene
An isotactic polypropylene and functionalized technology, applied in the field of regular polypropylene, can solve the problems of unfavorable use of terminal hydroxyl reaction, high molecular weight of polypropylene, large amount of MAO, etc., and achieve high reactivity and high terminal hydroxyl end capping rate. , the preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Catalyst: TiCl 4 / MgCl 2
[0039] Cocatalyst: Triethylaluminum
[0040] Chain transfer agent: diethyl zinc
[0041] External electron donor: cyclohexylmethyldimethoxysilane
[0043] Under anhydrous and oxygen-free conditions, 50mL of solvent, cocatalyst triethylaluminum (Al:Ti=60:1, molar ratio), external electron donor cyclohexylmethyldimethoxysilane (Si:Ti= 5:1, molar ratio) and chain transfer agent diethylzinc (Zn:Ti=3:1, molar ratio) were added to the Schlenk bottle, and finally 40 mg of the main catalyst was added, and propylene with a pressure of 0.1 MPa was introduced to polymerize The temperature is 60°C, and the polymerization time is 0.5h. After the polymerization is completed, dry oxygen is introduced for oxidation, and the reaction is carried out at 100°C for 0.5h. Then add 5 mL of 1mol / L hydrochloric acid aqueous solution to quench and deactivate the catalyst. After stirring for 5 min, stand to separate the liquid with a sepa...
Embodiment 2
[0045] Other experimental conditions are the same as in Example 1, Zn:Ti=5 (molar ratio). 3.16 g of isotactic polypropylene having a hydroxyl group at one end was obtained. The number average molecular weight of the polymer is 16.3×10 3 mol / g, the molecular weight distribution index is 7.7, the melting point is 159.6°C, and the hydroxyl end capping ratio is 0.73.
Embodiment 3
[0047] Other experimental conditions are the same as in Example 1, Zn:Ti=10 (molar ratio). 2.15 g of isotactic polypropylene having a hydroxyl group at one end were obtained. The number average molecular weight of the polymer is 8.2×10 3 mol / g, the molecular weight distribution index is 7.0, the melting point is 157.5°C, and the terminal hydroxyl end-capping rate is 0.75.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com