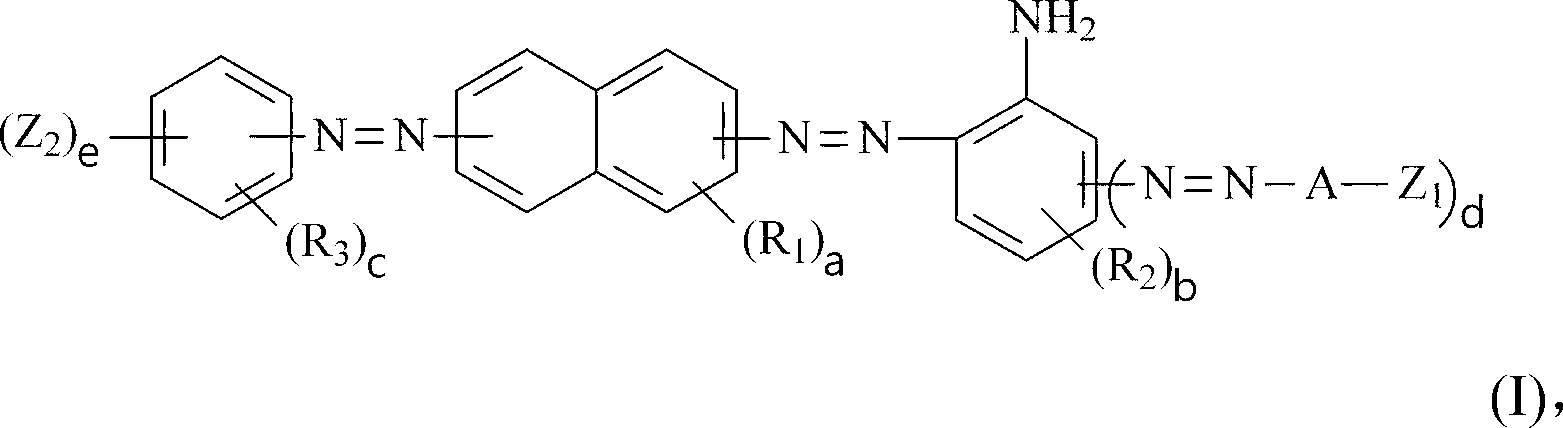
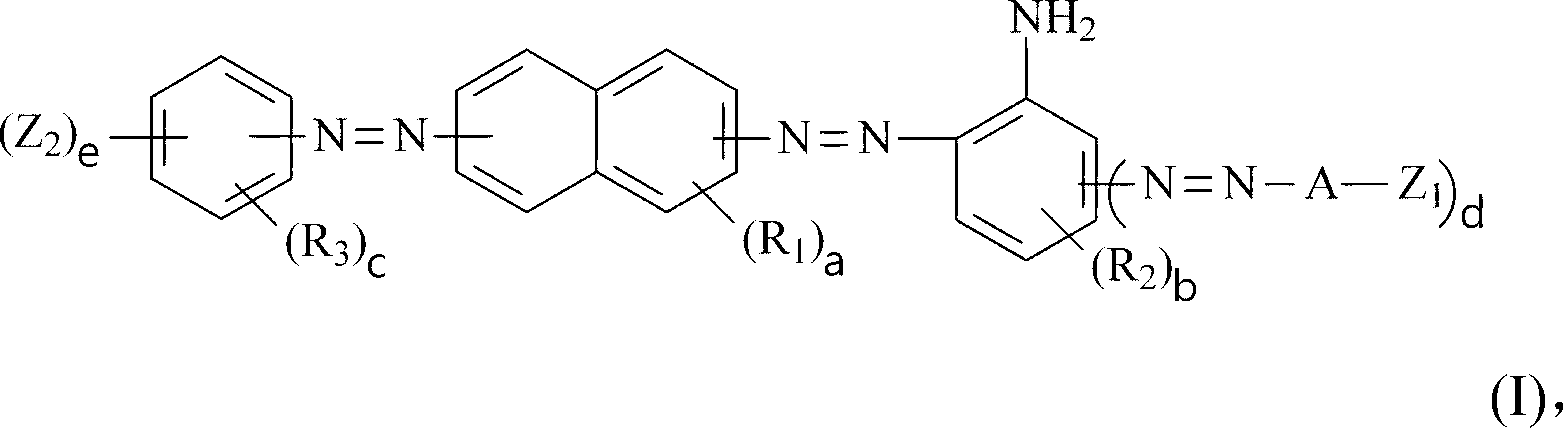
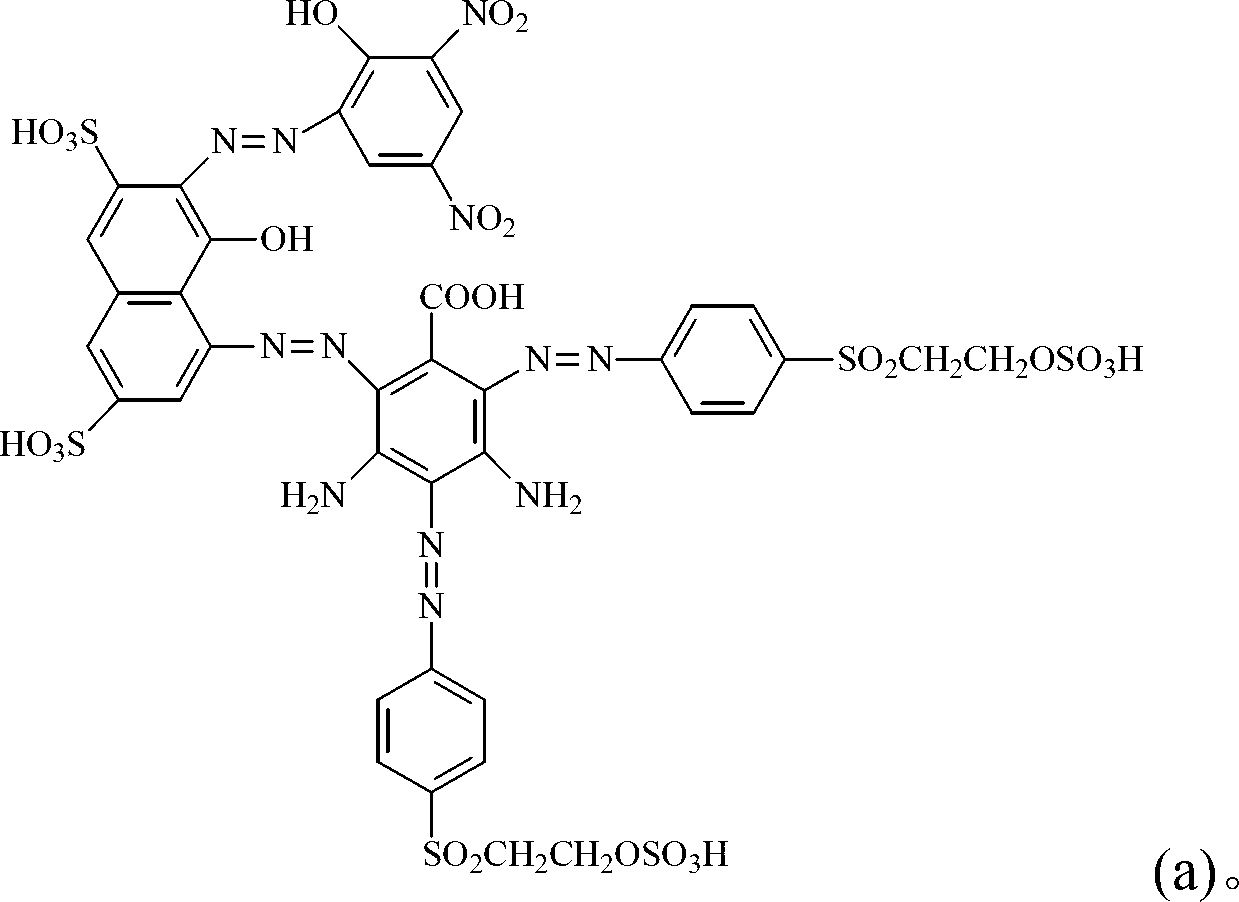
Dark brown reactive dye
A compound and selected technology, applied in the direction of reactive dyes, azo dyes, organic dyes, etc., can solve the problem of inability to meet the needs of high color strength and high color fastness, and achieve good dyeing reproducibility and good color fastness. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] (a) disperse 31.9 parts by weight of 4-amino-5-naphthol-2,7-disulfonic acid (4-amino-5-hydroxy-2,7-naphthalenedisulfonic acid) in 100 parts by weight of water, with 45 % liquid caustic soda to adjust the pH to be 7 to 9 to dissolve, add ice to cool to 0°C, add 34 parts by weight of 32% hydrochloric acid aqueous solution to disperse for 30 minutes, add 7.2 parts by weight of sodium nitrite, and control the temperature at 0 to 5°C until The diazotization reaction is complete, and excess sodium nitrite is removed with urea for later use.
[0107] (b) Get 15.2 parts by weight of 3,5-diaminobenzoic acid (3,5-diaminobenzoic acid) and 200 parts by weight of water to fully disperse, add to the above (a) reaction solution, add sodium carbonate to slowly adjust the reaction pH value Reach 3~7, after reacting for 3 hours, a brown product is obtained for future use.
[0108] (c) At 0°C, add 56.2 parts by weight of p-(β-sulfatoethylsulfone) aniline (4-(β-sulfatoethylsulfone) anilin...
Embodiment 2
[0112] (a), (b): Carry out the steps as (a) and (b) in Example 1.
[0113] (c) At 0°C, add 62.2 parts by weight of 2-amino-1-methoxy-4-(β-ethyl sulfate sulfone group) benzene (2-amino-1-methoxy- 4-(β-sulfatoethylsulfonyl)benzene) and 51.2 parts by weight of 32% hydrochloric acid aqueous solution, after fully stirring to form a dispersed solution, add 14.4 parts by weight of sodium nitrite, and control the temperature at 0 ~ 5 ° C until the diazotization reaction complete, add the reaction solution obtained in (b) above, slowly adjust the reaction pH value to 4-8 with sodium carbonate, and obtain a brown product after reacting for 3 hours.
[0114] (d) Get 19.9 parts by weight of 2-amino-4,6-dinitrophenol, stir and disperse in 100 parts by weight of water, add 34 parts by weight of 32% aqueous hydrochloric acid, add 7.2 parts by weight of sodium nitrite, and control the At a temperature of 0-5°C, until the diazotization reaction is complete, remove excess sodium nitrite with u...
Embodiment 3
[0117] (a), (b): Carry out the steps as (a) and (b) in Example 1.
[0118] (c) at 0°C, add 56.2 parts by weight of m-(β-sulfatoethylsulfone) aniline (3-(β-sulfatoethylsulfone) aniline) and 51.2 parts by weight of 32% hydrochloric acid aqueous solution in 600 parts by weight of water, fully After stirring to form a dispersed solution, add 14.4 parts by weight of sodium nitrite, and control the temperature at 0 to 5°C until the diazotization reaction is complete, add the reaction solution obtained in (b) above, and slowly adjust the reaction pH with sodium carbonate The value reaches 4-8, and the brown product is obtained after reacting for 3 hours.
[0119] (d) Get 19.9 parts by weight of 2-amino-4,6-dinitrophenol, stir and disperse in 100 parts by weight of water, add 34 parts by weight of 32% aqueous hydrochloric acid, add 7.2 parts by weight of sodium nitrite, and control the At a temperature of 0-5°C, until the diazotization reaction is complete, remove excess sodium nitri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com