Blue organic light emitting materials o-pyridine condensed 2,6-substitued monoamine mercury complexes and preparation method thereof
A technology of luminescent materials and complexes, applied in luminescent materials, mercury-organic compounds, chemical instruments and methods, etc., can solve the problems of poor brightness, low luminous efficiency, short life, etc., and achieve good monochromatic performance, low cost, and stability. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
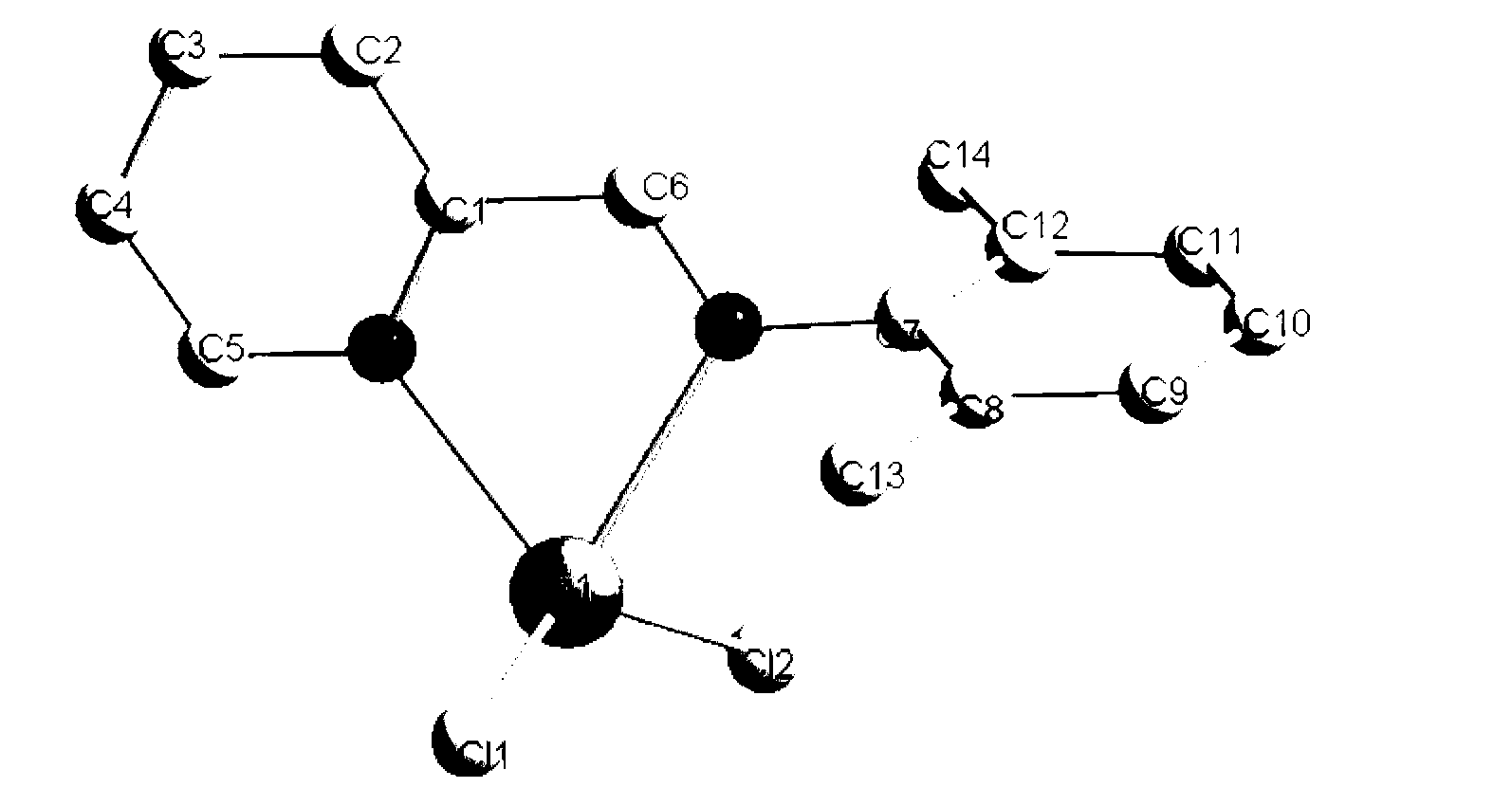
[0026] Specific implementation mode 1: The blue organic light-emitting material in this implementation mode is [(2,6-dimethylphenyl)-pyridine-2-methyleneamine] with the 2,6-substituted monoamine mercury complex HgCl 2 , [(2,6-Dimethylphenyl)-pyridine-2-methyleneamine]HgCl 2 The molecular formula is C 14 h 14 Cl 2 N 2 Hg, whose structural formula is
[0027]
specific Embodiment approach 2
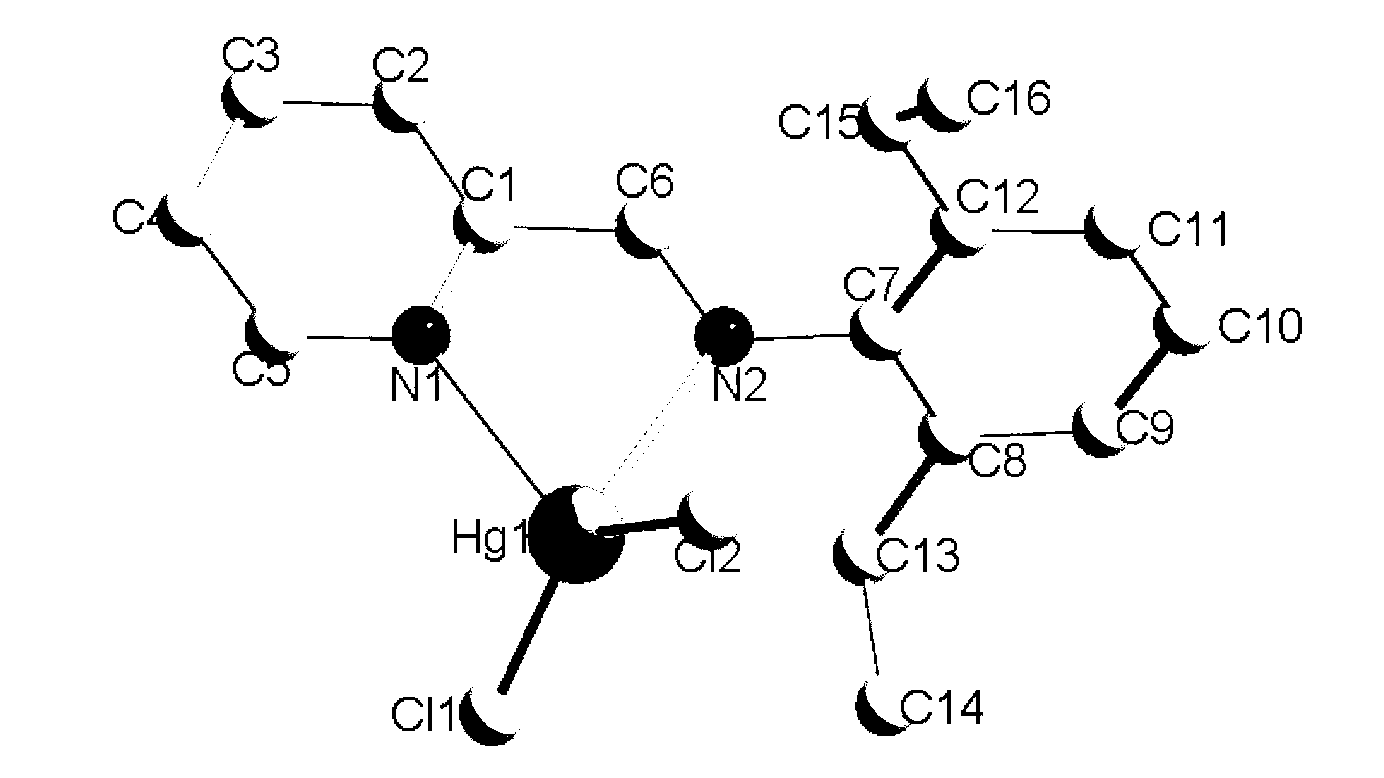
[0028] Specific embodiment 2: In this embodiment, the blue organic luminescent material is [(2,6-diethylphenyl)-pyridine-2-methyleneamine], which is a mercury complex substituted by 2,6-position monoamines in the blue organic luminescent material. HgCl 2 , [(2,6-Diethylphenyl)-pyridine-2-methyleneamine]HgCl 2 The molecular formula is C 16 h 18 Cl 2 N 2 Hg, whose structural formula is
[0029]
specific Embodiment approach 3
[0030] Specific embodiment three: Specific embodiment one The preparation method of the blue organic luminescent material o-pyridine 2,6-substituted monoamine mercury complex is carried out according to the following steps:
[0031] 1. Under the condition of nitrogen protection, mix 20-30 mg of (2,6-dimethylphenyl)-pyridine-2-methyleneamine and 25-40 mg of HgCl 2 Dissolve in a mixed organic solvent, heat to reflux, and stir for 7 hours to obtain a yellow reaction liquid;
[0032] 2. Filter the product of step 1 while it is hot, and then volatilize at room temperature for 3 to 10 days to obtain [(2,6-dimethylphenyl)-pyridine-2-methyleneamine]HgCl 2 ;
[0033] The mixed organic solvent described in step 1 is CH 3 OH and CH 2 Cl 2 A mixed solution of which CH 3 OH and CH 2 Cl 2 The volume ratio is 1:5.
[0034] The reaction equation of the present embodiment is as follows
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com