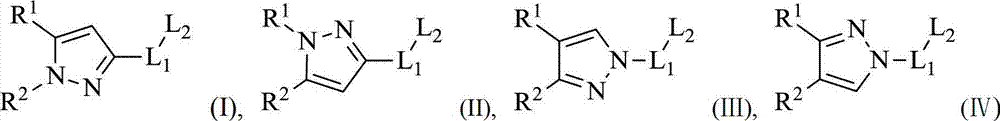
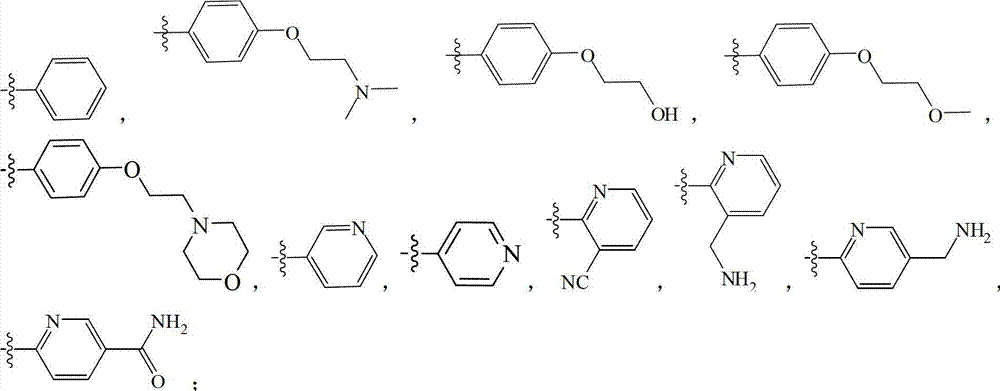
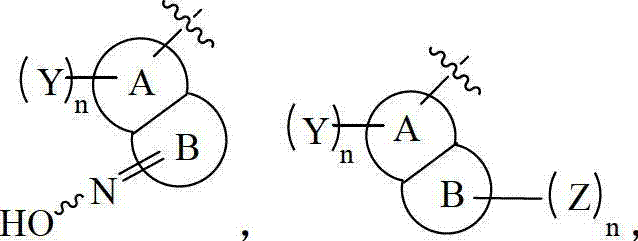Substitutional pyrazol kinase inhibitor
An alkyl and heterocyclic group technology, applied in the field of substituted pyrazole kinase inhibitors, can solve problems such as poor selectivity and insufficient activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] (1) Preparation of TM1
[0102] In the three-necked flask, add 5-bromo-2,3-dihydro-1H-inden-1-one O-methyloxime SM1 (prepared with reference to US2007 / 99954A1 (2007)), freshly distill tetrahydrofuran, and store in a dry ice bath at -75°C Under the same conditions, n-butyllithium (1.4 equivalents) was added with a syringe, and the reaction was stirred for 0.5 hours. Add isopropyl borate (1.4 eq) and continue to stir the reaction, then remove the dry ice bath, the mixture is slowly raised to room temperature, and stir the reaction. Then 2M hydrochloric acid was added and the reaction was stirred. After the reaction, saturated sodium chloride water was added, the liquid was separated, the organic phase was dried, concentrated under reduced pressure, separated and purified by silica gel column chromatography to obtain the intermediate TM1.
[0103] (2) Preparation of TM2
[0104] Weigh isonicotinic acid methyl ester SM3 and add it into an appropriate amount of tetrahydro...
experiment example 1
[0127] Experimental Example 1 The in vitro enzymatic activity test of the compound of the present invention
[0128] Need testing product: the compound 2 that embodiment 2 prepares
[0129] Reference substance: SB-590885, commercially available
[0130] experimental method:
[0131] The meanings of the English and English abbreviations in the following tests are as follows:
[0132] HEPES: Hydroxyethylpiperazineethanesulfonic acid;
[0133] Brij-35: lauryl polyethylene glycol ether;
[0134] EDTA: ethylenediaminetetraacetic acid;
[0135] Fluorescein-MAP2K1: Fluorescein-labeled MAP2K1;
[0136] ATP: adenosine triphosphate;
[0137] DMSO: dimethyl sulfoxide;
[0138] MgCl 2 : Magnesium chloride.
[0139] 1. Preparation of test reagents
[0140] ① 1X Kinase Buffer (50mM HEPES, PH7.5, 10mM MgCl 2 , 1mM EGTA, 0.01%Brij-35);
[0141] ② 2-fold kinase solution (add corresponding kinase to 1-fold kinase buffer to prepare 2-fold kinase solution, the final concentration is ...
experiment example 2
[0157] Experimental Example 2 Pharmacokinetics of the compounds of the present invention in male SD rats
[0158] Need testing product: the compound 2 that embodiment 2 prepares
[0159] Control drug: SB-590885, commercially available
[0160] (SB-590885)
[0161] Experimental Animals Male SD rats, 3 / administration route / compound, body weight 220-250g.
[0162] Dissolution scheme
[0163] Compound 2 of the present invention: 1%DMSO+15%HP-β-CD solution+84% normal saline.
[0164] Control drug SB-590885: 1%DMSO+10%HP-β-CD solution+89% normal saline;
[0165] experimental method
[0166]Administration The test product is administered by intravenous injection (IV), the dosage is 1mg / kg, and the volume of administration is 1mL / kg; Volume 2mL / kg.
[0167] 0.083 hours, 0.25 hours, 0.5 hours, 1 hour, 2 hours, 4 hours, 6 hours, 8 hours, 24 hours after administration of blood collection; 0.17 hours, 0.5 hours, 1 hour, 2 hours, At 4 hours, 6 hours, 8 hours, and 24 hours, about ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com