Lovastatin enteric coated sustained-release pellet capsule and preparation method thereof
A technology of sustained-release pellets and lovastatin, which is applied in the fields of pharmaceutical formulations, metabolic diseases, bulk delivery, etc. It can solve the problems of not having enteric properties, and achieve small differences in individual bioavailability, good absorption, and not easy to compress broken effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
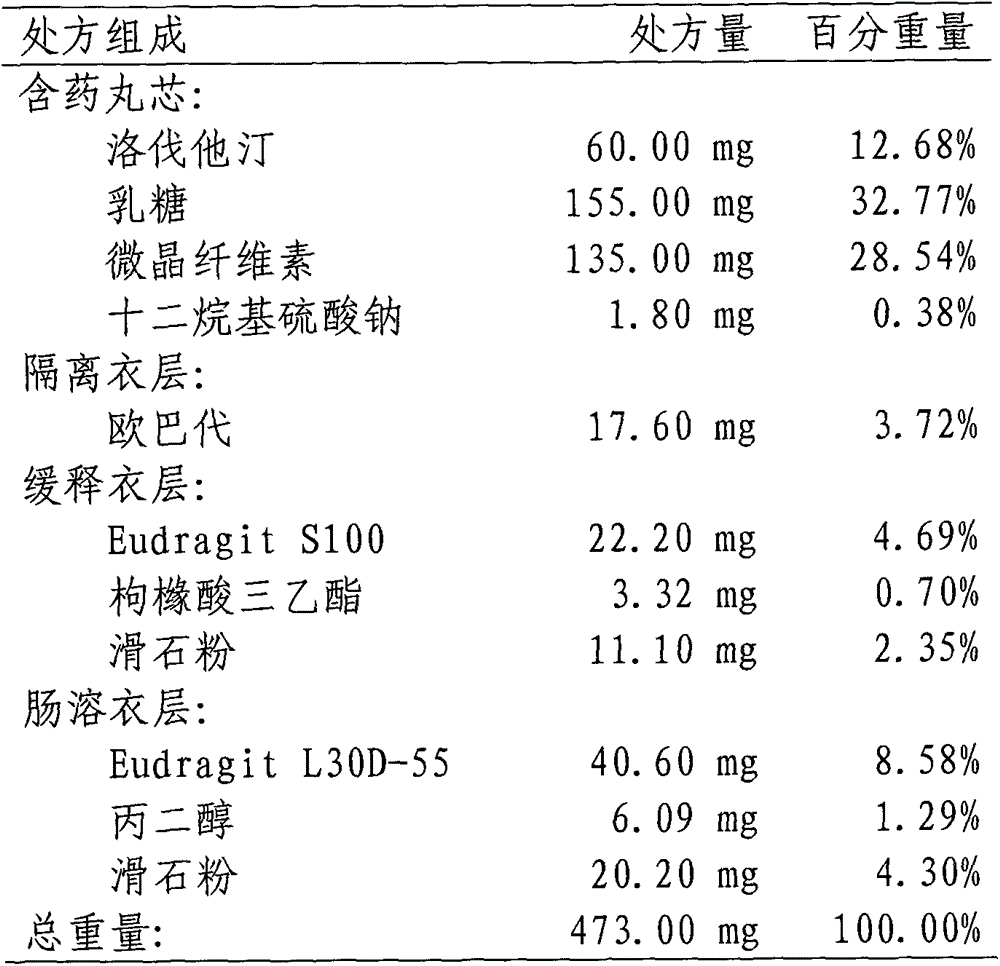
[0039] 1. Prescription:
[0040]
[0041] 2. Preparation process:
[0042] (1) Preparation of pill-containing core: the micronized lovastatin, lactose, and microcrystalline cellulose are mixed uniformly in equal increments, sodium lauryl sulfate is dissolved in water to make a diluted solution, and then added to the mixed powder. The soft material is obtained, pellets are extruded and spheronized, dried in an oven at 60°C, and sieved to select 20-40 mesh-containing pellet cores.
[0043] (2) Coating isolation layer: Dissolve Opadry in water to prepare a 15% solid content coating solution, place the pill-containing core in a fluidized bed for coating, and then coat the pill with isolation layer at 50°C Oven drying.
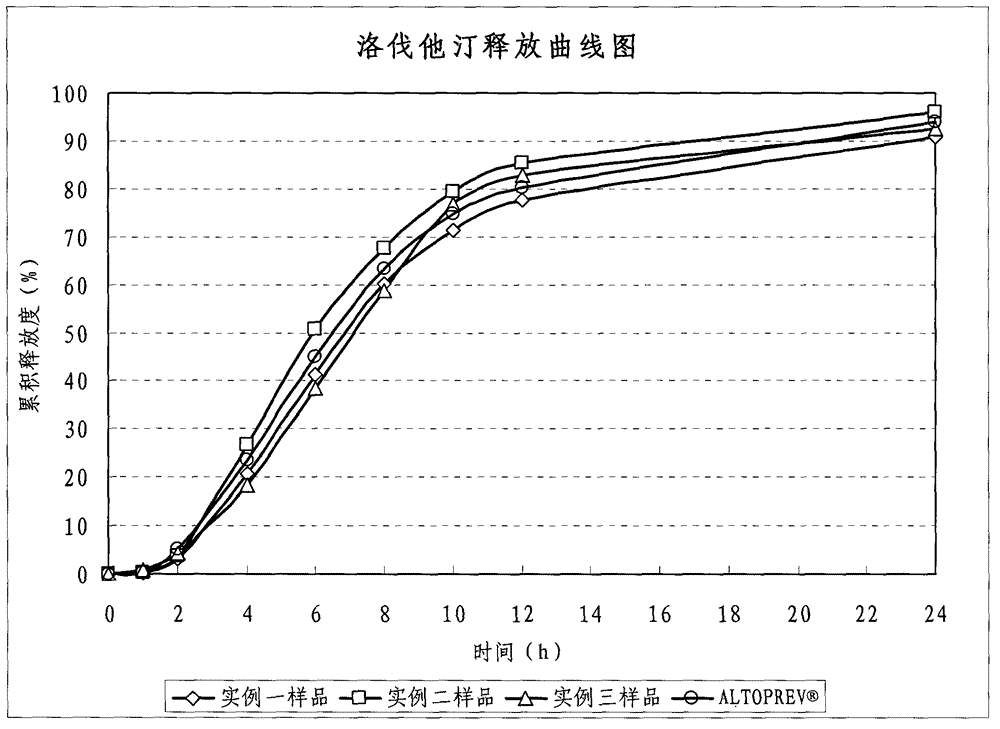
[0044] (3) Coating slow-release coating layer: Dissolve Eudragit S100, triethyl citrate and talc in absolute ethanol to prepare a coating solution with a solid content of 12%, and place the plain pills in a fluidized bed for coating. The coated and sustained-release pell...
Embodiment 2
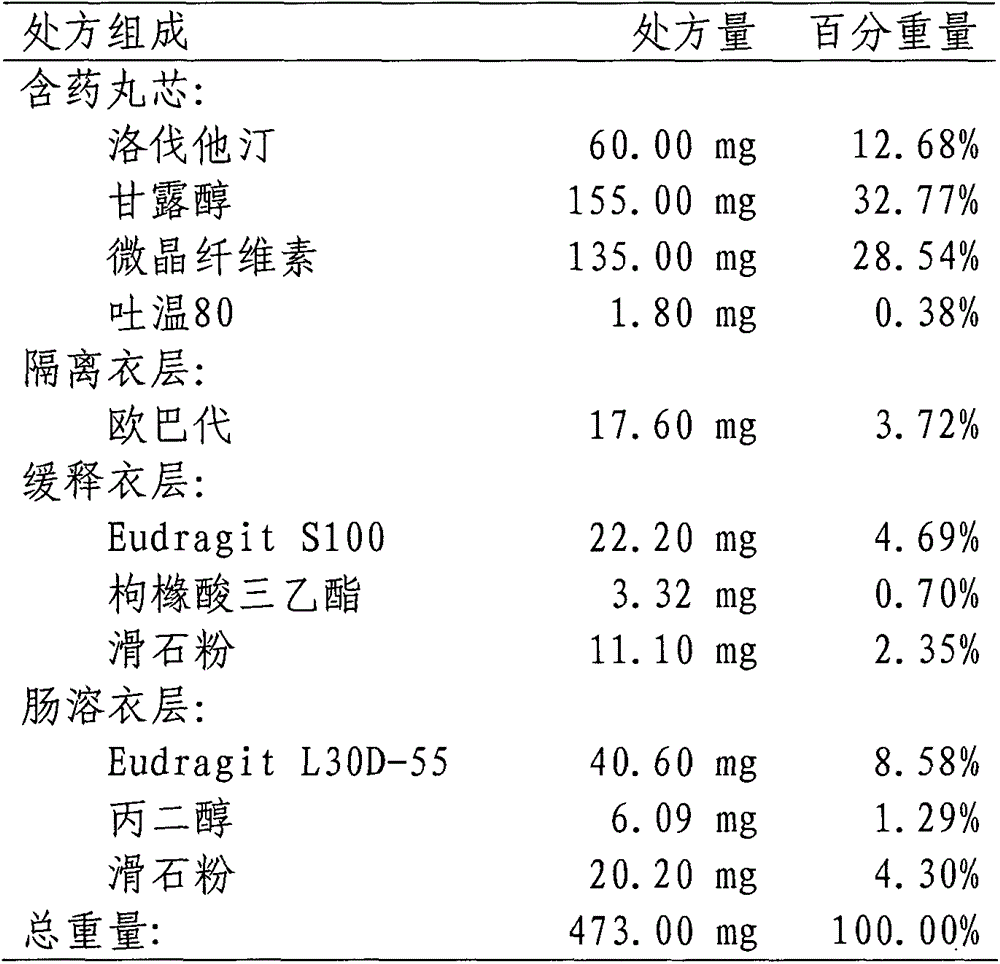
[0048] 1. Prescription:
[0049]
[0050] 2. Preparation process:
[0051] (1) Preparation of pill-containing core: the micronized lovastatin, lactose, and microcrystalline cellulose are mixed uniformly in equal increments, sodium lauryl sulfate is dissolved in water to make a diluted solution, and then added to the mixed powder. The soft material is obtained, pellets are extruded and spheronized, dried in an oven at 60°C, and sieved to select 20-40 mesh-containing pellet cores.
[0052] (2) Coating isolation layer: Dissolve Opadry in water to prepare a 15% solid content coating solution, place the pill-containing core in a fluidized bed for coating, and then coat the pill with isolation layer at 50°C Oven drying.
[0053] (3) Coating slow-release coating layer: Dissolve Eudragit S100, triethyl citrate and talc in absolute ethanol to prepare a coating solution with a solid content of 12%, and place the plain pills in a fluidized bed for coating. The coated and sustained-release pell...
Embodiment 3
[0057] 1. Prescription:
[0058]
[0059] 2. Preparation process:
[0060] (1) Preparation of pill-containing core: Dissolve and disperse micronized lovastatin and sodium lauryl sulfate in water to make a drug-containing solution, and place the blank pill core in a fluidized bed to adjust the process parameters to ensure fluidization State, spray the above-mentioned drug-containing solution on the surface of the pellet core, dry, and select 20-40 mesh pellet cores.
[0061] (2) Coating isolation layer: Dissolve Opadry in water to prepare a 15% solid content coating solution, place the pill-containing core in a fluidized bed for coating, and then coat the pill with isolation layer at 50°C Oven drying.
[0062] (3) Coating the sustained-release coating layer: Dissolve Eudragit NE30D and talc in water to prepare a 15% solid content coating solution. Place the pellet core in a fluidized bed for coating. Dry and mature in an oven at ℃ for 24 hours.
[0063] (4) Enteric coating layer: Diss...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com