Novel self-hydrogen-supplying diphenyl ketone macromolecular photoinitiator and preparation method thereof
A technology of benzophenone and photoinitiator, which is applied in the field of preparation of new self-supplying hydrogen benzophenone macromolecular photoinitiator, can solve the problems of low ultraviolet absorption and poor initiation activity, so as to improve compatibility and reduce Volatility and Migration, Effect of Reducing Toxicity and Surface Migration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
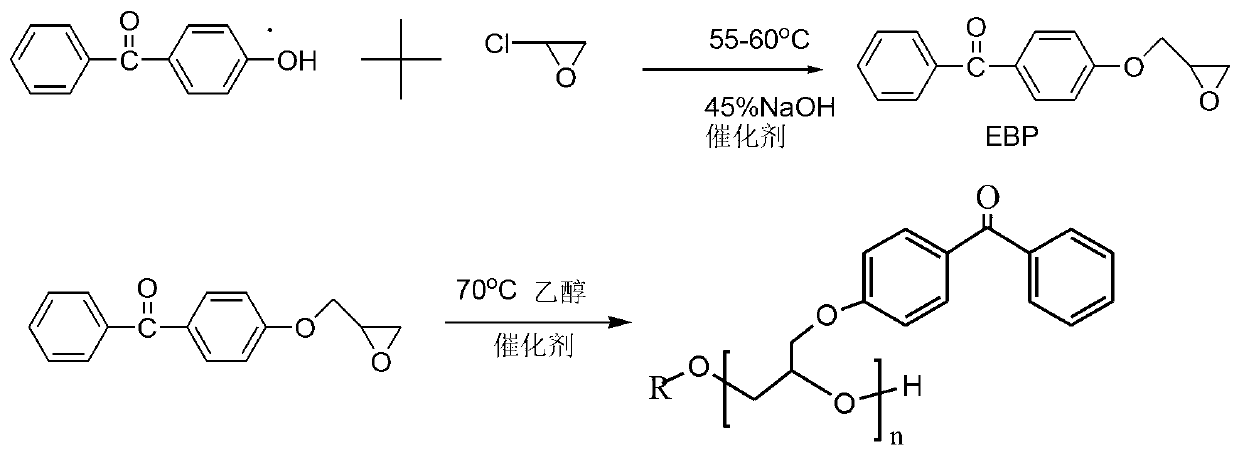
[0017] Weigh 10g of 4-(2,3-epoxypropoxy)benzophenone (EBP) and dissolve it in 20ml of ethanol solvent, dissolve 1g of potassium tert-butoxide in 10ml of ethanol, and dissolve 4-(2 Add the ethanol solvent of 3-glycidoxy) benzophenone (EBP) into the four-necked flask, heat and stir, raise the temperature to 80°C, and start adding the ethanol solution with the catalyst dropwise, and control the dropping rate for about 1 hour. , the temperature was controlled at 85°C, cooled to room temperature after 7 hours of reaction, dissolved in dichloromethane, washed twice with 10 mL of 2% NaOH solution and deionized water, dried overnight with anhydrous sodium sulfate, filtered, and removed by rotary evaporation Dichloromethane was vacuum-dried to obtain a novel self-supplying hydrogen benzophenone macromolecular photoinitiator. The structure is shown in structural formula 1: 1 H NMR (500MHz) in CDCl 3 : δ1.21ppm (9H, CH 3 ), 3.64-4.22ppm (nH, n>2; CH 2 ), 4.05ppm (1H, CH), 4.81ppm (1H...
Embodiment 2
[0021] Weigh 10g of 4-(2,3-epoxypropoxy)benzophenone (EBP) and dissolve it in 20ml of ethanol solvent, dissolve 2g of potassium tert-butoxide in 10ml of ethanol, dissolve 4-( Add the ethanol solvent of 2,3-glycidyloxypropoxy)benzophenone (EBP) into the four-necked flask, heat and stir, and start adding the ethanol solution with the catalyst dropwise when the temperature rises to 80°C, and add dropwise at a controlled rate for about 1 hour Completed, the temperature was controlled at 85°C, cooled to room temperature after 7 hours of reaction, dissolved in dichloromethane, washed twice with 10mL of 2% NaOH solution and deionized water, dried overnight with anhydrous sodium sulfate, filtered, and rotary evaporated The methylene chloride was removed, and vacuum drying was carried out to obtain a novel self-supplying hydrogen benzophenone macromolecular photoinitiator. The structure is shown in structural formula 1: 1 H NMR (500MHz) in CDCl 3 : δ1.21ppm (9H, CH 3 ), 3.64-4.22ppm...
Embodiment 3
[0023] Weigh 10g of 4-(2,3-epoxypropoxy)benzophenone (EBP) and dissolve it in 20ml of ethanol solvent, dissolve 1g of sodium tert-butoxide in 10ml of ethanol, and dissolve 4-(2 Add the ethanol solvent of 3-glycidoxy) benzophenone (EBP) into the four-necked flask, heat and stir, raise the temperature to 80°C, and start adding the ethanol solution with the catalyst dropwise, and control the dropping rate for about 1 hour. , the temperature was controlled at 85°C, cooled to room temperature after 7 hours of reaction, dissolved in dichloromethane, washed twice with 10 mL of 2% NaOH solution and deionized water, dried overnight with anhydrous sodium sulfate, filtered, and removed by rotary evaporation Dichloromethane was vacuum-dried to obtain a novel self-supplying hydrogen benzophenone macromolecular photoinitiator. The structure is shown in Structural Formula 1: H NMR (500MHz) in CDCl 3 : δ1.26ppm (9H, CH 3 ), 3.64-4.22ppm (nH, n>2; CH 2 ), 4.15ppm (1H, CH), 4.78ppm (1H, OH),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com