Preparation method of azilsartan polymorphism
A technology of crystal and dihydrogen, applied in the field of preparation of polymorphs of azilsartan, can solve problems such as undisclosed crystal preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
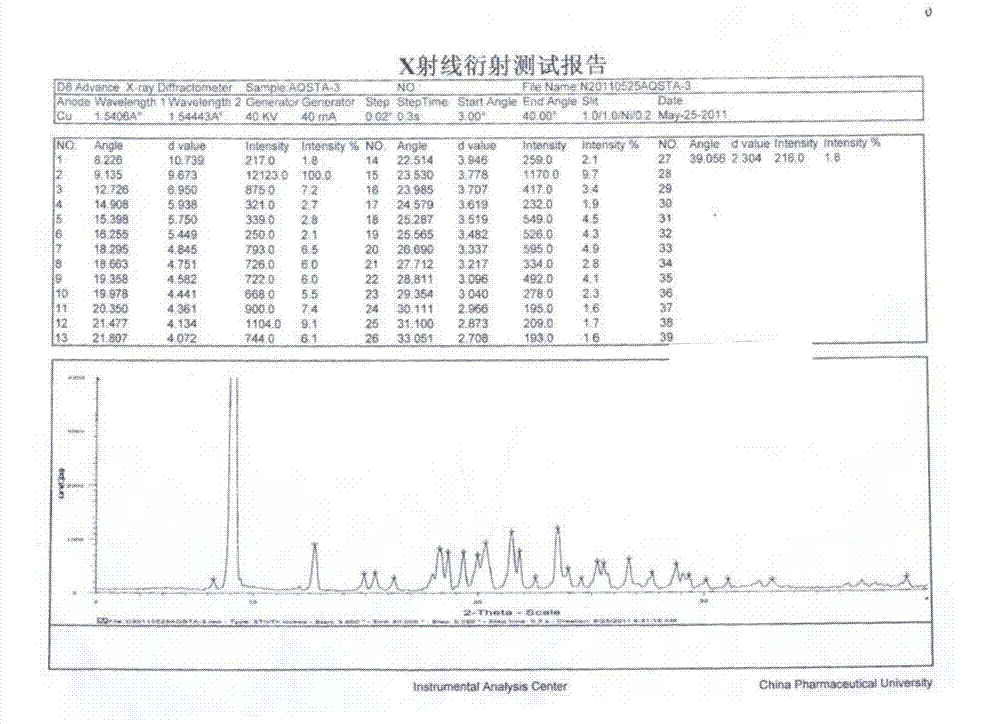
[0067]Add 10g of azilsartan to 35ml of DMF, heat to 70°C to dissolve the solid, then suction filter while hot, transfer the filtrate to a round bottom flask at 70°C for 10min, then slowly add 40ml of purified water dropwise at this temperature. As the dropwise addition proceeded, a white solid gradually precipitated out. After the dropwise addition was completed, the oil bath was lowered to 50° C. and stirred overnight. Suction filtration while it was hot, the obtained solid was washed with hot water (20mlX3) and ethanol (15mlX2), and vacuum-dried at 50°C for 15 hours to obtain 7.9g of azilsartan type I crystalline white powder with a yield of 79% and a melting point of 200.9°C.
Embodiment 2
[0069] Add 10g of azilsartan into 20ml of ethanol and 30ml of acetone, heat to 60°C, stir and beat for 1 hour, then naturally cool to 20°C, continue to stir for 1 hour, then filter with suction, and vacuum dry the obtained solid at 50°C for 5 hours to obtain azilsartan II Type crystal white powder 8.4g, yield 84%, melting point 162.8 ℃.
Embodiment 3
[0071] Add 10g of azilsartan to 30ml of DMSO, heat to 60°C to dissolve the solid, then suction filter while hot, transfer the filtrate to a round bottom flask, heat to 60°C and keep it for 10min, then slowly add 50ml of purified water. As the dropwise addition proceeded, a white solid gradually precipitated out. After the dropwise addition was completed, the oil bath was lowered to 40° C. and stirred overnight. Suction filtration while hot, the obtained solid was washed with hot water (20mlX3) and ethanol (15mlX2), and vacuum dried at 50°C for 15h to obtain 6.8g of azilsartan type I crystalline white powder, yield 68%, melting point 199.8°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com