Preparation method of phloroglucinol
A technology of phloroglucinol and dichlorophenol, which is applied in the field of preparation of phloroglucinol, can solve problems such as inability to realize industrialized production, consume a large amount of solvent, and difficult sources of raw materials, and achieve reduced processing difficulty, less solvent loss, Easy to prepare
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
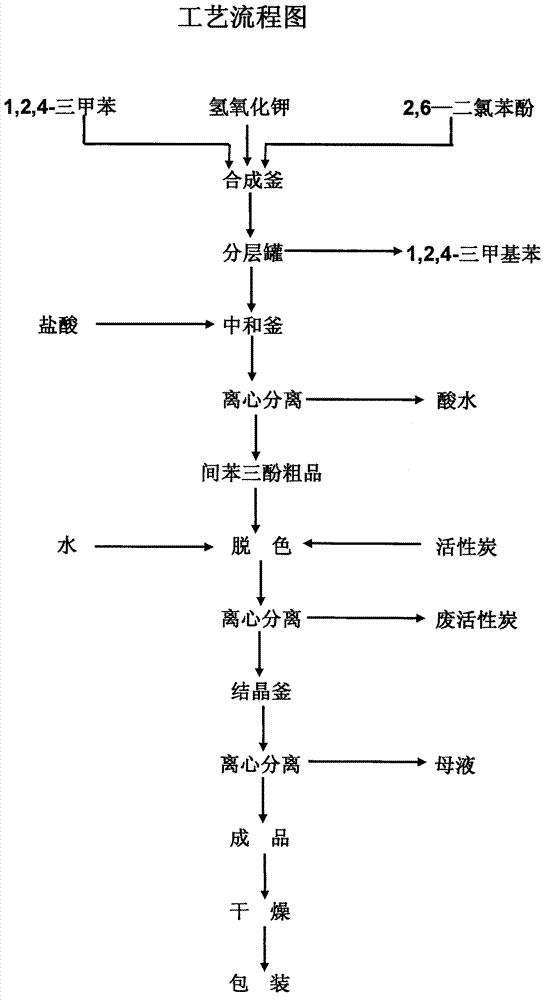
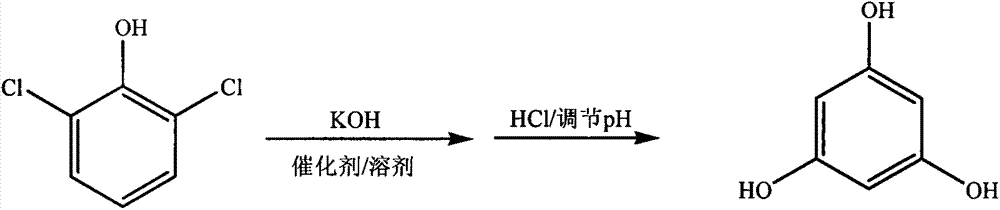
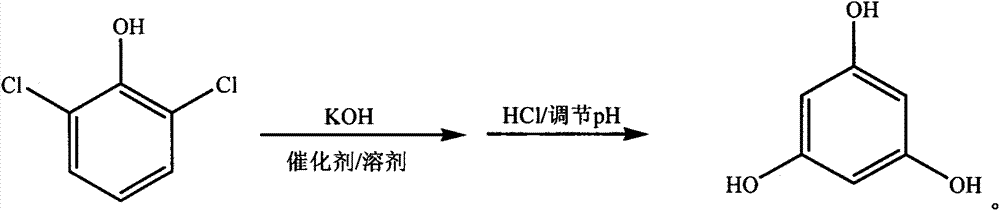
[0021] In a 2000mL four-neck flask, add 1.0mol (163.0g) 2,6-dichlorophenol, 8.0mol (448.8g) potassium hydroxide, 0.163g cuprous chloride, 815.0g (930.6mL) 1,2, 4-Trimethylbenzene, heat and keep the bath temperature at 170°C, mix and stir for 17 hours, transfer to a separatory funnel after cooling to room temperature, add 652.0g of water, stand to separate layers, separate the upper solvent, the lower layer The water phase is adjusted to a pH of 0.5 with 37% hydrochloric acid by mass percentage, and the acid water is separated by centrifugation. The crude phloroglucinol is dissolved in hot water, decolorized by activated carbon, and centrifuged to separate the waste activated carbon. The mother liquor is recrystallized, centrifuged, and separated. The mother liquor is produced, and the refined product is dried and pulverized to obtain the finished product with a yield of 68%.
Embodiment 2
[0023] In a 2000mL four-necked flask, add 1.0mol (163.0g) 2,6-dichlorophenol, 9.0mol (504.9g) potassium hydroxide, 1.63g copper sulfate, 978.0g (1116.7mL) 1,2,4- Trimethylbenzene, heating and keeping the bath temperature at 180 °C, mixed and stirred for 14 hours, cooled to room temperature, transferred to a separatory funnel, added 815.0 g of water, allowed to stand for layers, separated the upper solvent, and the lower water phase The pH was adjusted to 3.0 with 31% hydrochloric acid by mass percentage, and the remaining steps were the same as in Example 1, and the yield was 71%.
Embodiment 3
[0025] In a 2000mL four-neck flask, add 1.0mol (163.0g) 2,6-dichlorophenol, 8.5mol (476.9g) potassium hydroxide, 0.83g cuprous chloride, 896.5g (1023.5mL) 1,2, 4-Trimethylbenzene, heat and keep the bath temperature at 175°C, mix and stir for 15 hours, transfer to a separatory funnel after cooling to room temperature, add 733.5g of water, stand to separate layers, separate the upper solvent, the lower layer The pH of the aqueous phase was adjusted to 1.5 with 35% hydrochloric acid by mass percentage, and the rest of the steps were the same as in Example 1, and the yield was 69%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com