Cross-blood-brain-barrier targeting multimodal nano-medicine used in brain tumor diagnosis
A nano-drug, multi-modal technology, which is applied in the multi-modal nano-drug targeting across the blood-brain barrier, the synthesis of magnetic/fluorescent nano-diagnostic drugs and their intermediates, and the application in in vivo non-invasive brain tumor multi-modal imaging , In the field of diagnostic drugs, it can solve the problems that have not yet been reported on multimodal nanodiagnostic drugs with cross-BBB secondary targeting, and achieve the best tumor tracing effect and improve the effect of targeting efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Synthesis of Cysteine-modified Polypeptide Angiopep-2
[0056] In order to connect Angiopep-2 to G5 without affecting the specificity of receptor binding, we synthesized Angiopep-2 modified with a cysteine residue at the C-terminus by Boc-protected solid-phase peptide synthesis method: TFFYGGSRGKRNNFKTEEYC( MW = 2402 Da). The resulting entire protected linear peptide chain is
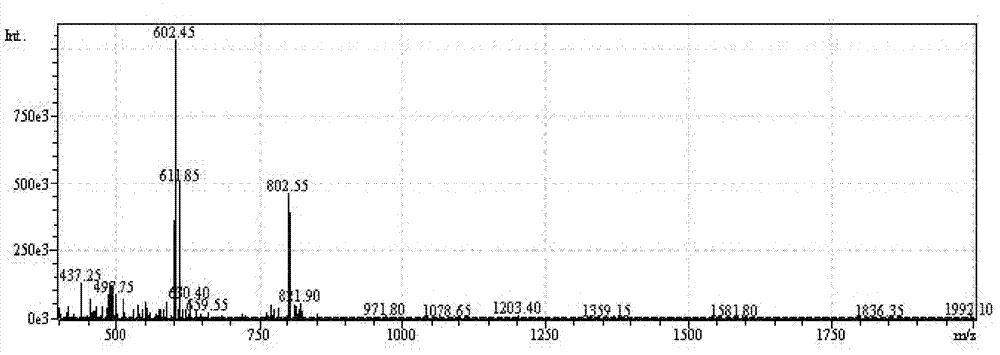
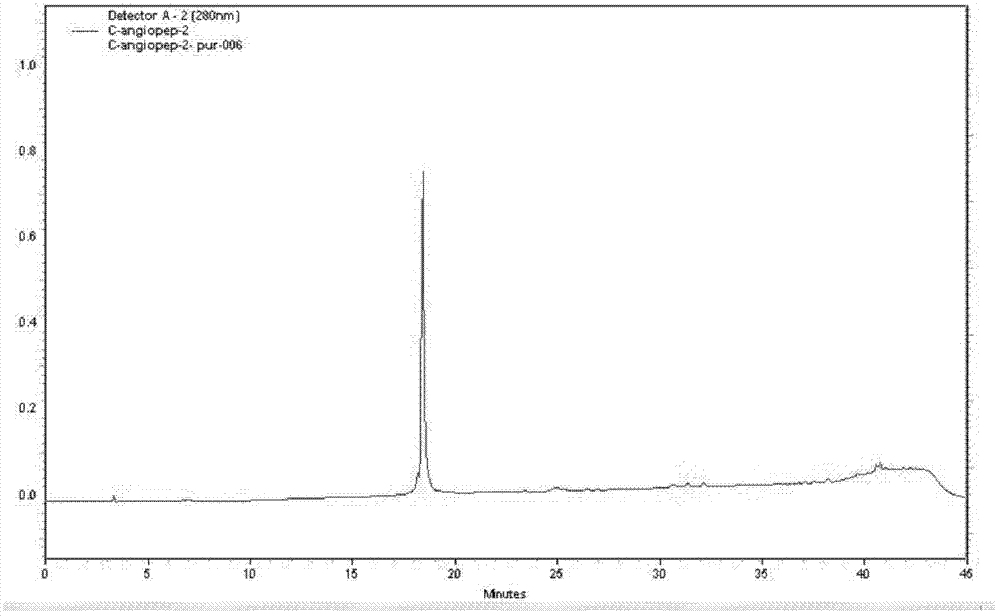
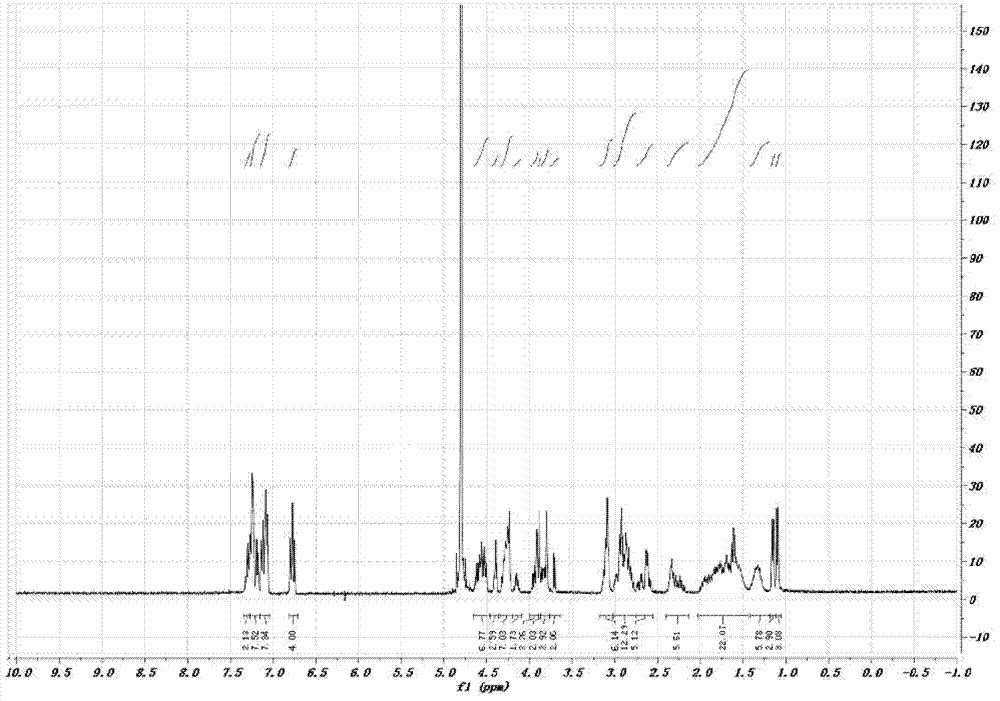
[0057] H-Thr(Bzl)-Phe-Phe-Tyr(Br-Z)-Gly-Gly-Ser(Bzl)-Arg(Tos)-Gly-Lys(Cl-Z)-Arg(Tos)-Asn(Xan) -Asn(Xan)-Phe-Lys(Cl-Z)-Thr(Bzl)-Glu(OcHex)-Glu(OcHex)-Tyr(Br-Z)-Cys(PMeBzl)-OH. Deprotected with HF, purified by preparative HPLC, lyophilized. The purity of the product was confirmed by analytical HPLC. There is a single peak at 802.5 [M3+] in ESI-MS with a calculated molecular weight of 2404.6 [M+H+]. ESI-MS and analytical HPLC results are attached figure 1 , 2 . C-Angiopep-2 1 See attached for H NMR spectrum image 3 .
Embodiment 2
[0059] Synthesis of compound 1
[0060] 3.5mg (1.0×10 -5 mol)c[RGDyK] cyclic peptide was dissolved in 300 μL of DMF, and 6 μL of triethylamine was added to mix well. Quickly add 300 μL of 8.2 mg (4.1×10 -6 mol) maleimide-PEG 2k -N-hydroxysuccinimide ester (Malemide-PEG 2k -NHS) in DMF solution. After stirring and reacting at room temperature for 2 hours, a PEG derivative of c[RGDyK] cyclic peptide was formed.
Embodiment 3
[0062] Synthesis of compound 2
[0063] The above reaction solution was directly added to 1.0mL containing 11.6mg (4×10 -7 mol) of dendrimer G5 in 1X PBS (pH 7.4) solution. After stirring at room temperature for 1 h, compound 2 was formed, which was purified by ultrafiltration (4000 rpm, 30 min×3) using a centrifugal filter tube with a molecular weight of 10 kDa. The molar ratio between G5, PEG and RGD is determined by their concentration in compound 2 1 The integral in the H NMR spectrum is calculated. About 6 c[RGDyK] cyclic peptides were labeled on each G5 molecule. Compound 2 1 See attached for H NMR spectrum Figure 4 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com