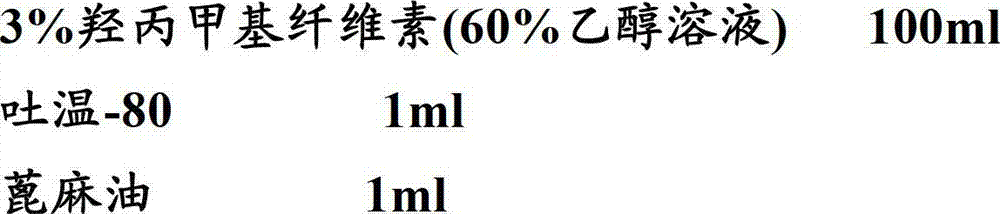Pharmaceutical composition containing lappaconitine and oxycodone
A kind of technology of clathrin and clathrate hydrobromide, which is applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1——tablet containing homogenate and oxycodone
[0063] prescription:
[0064] Tablets:
[0065]
[0066] Film Coating Prescription:
[0067]
[0068]
[0069] Preparation method: pass the prescribed amount of urine, oxycodone, microcrystalline cellulose and 200g starch through an 80-mesh sieve and mix evenly, use 10% PVP K-30 aqueous solution as a binder to make granules and dry, dry Mix the granules with the rest of dried starch and micropowder silica gel, and press plain tablets with different weights, each tablet contains 10mg / 10mg, 5mg / 5mg, or 2.5mg / 2.5mg of oxycodone . Then coat the plain tablet again until the weight of the tablet increases by 3-4%.
Embodiment 2
[0070] Embodiment 2——tablet containing homogenate and oxycodone
[0071] prescription:
[0072]
[0073] Preparation method: pass the prescribed amount of urine, oxycodone, microcrystalline cellulose and 200g starch through an 80-mesh sieve and mix evenly, use 10% PVP K-30 aqueous solution as a binder to make granules and dry, dry The granules are mixed with the rest of dried starch and micropowder silica gel, and tablets of different weights are compressed. Each tablet contains 5 mg / 10 mg, 2.5 mg / 5 mg, or 1.25 mg / 2.5 mg / oxycodone respectively mg.
Embodiment 3
[0074] Embodiment 3——tablet containing homogenate and oxycodone
[0075] prescription:
[0076]
[0077]
[0078] Preparation method: refer to the method of Example 2 to prepare granules and compress into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com